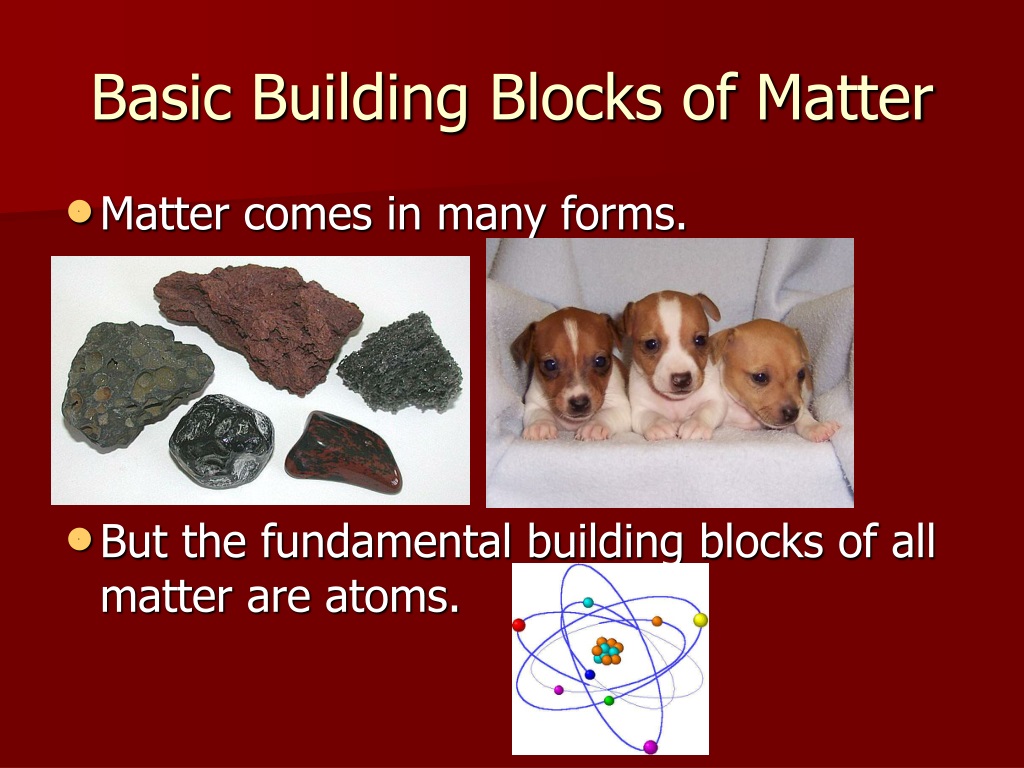
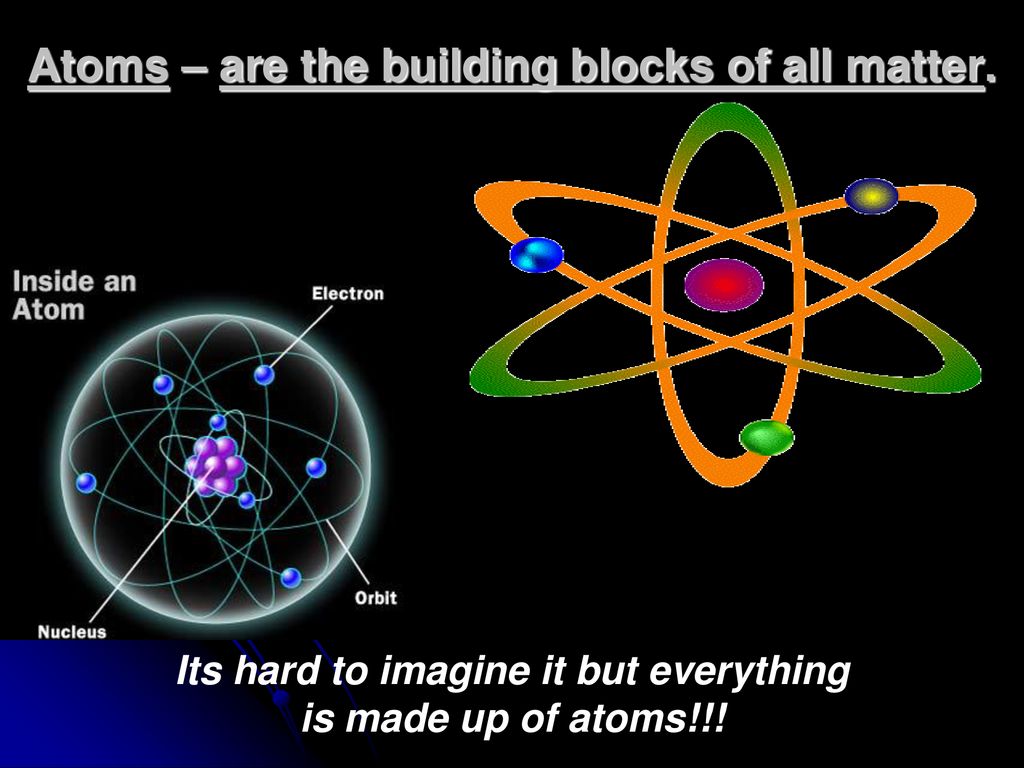
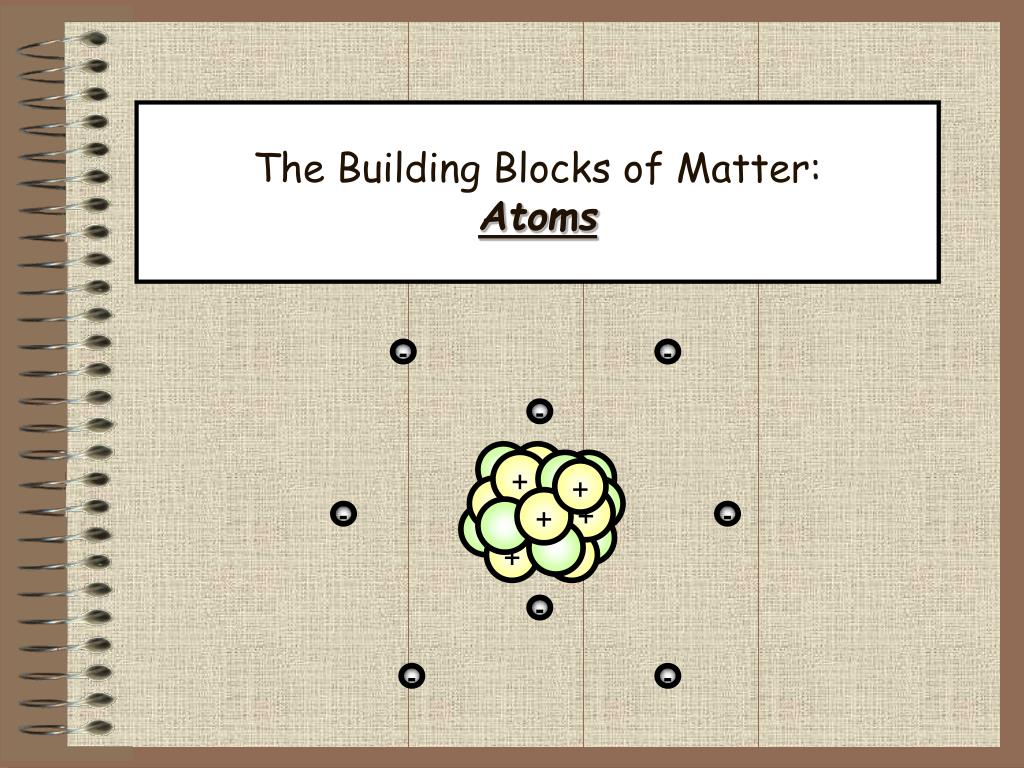
Basic Building Block Of All Matter
Basic Building Block Of All Matter - Additionally, we discuss isotopes and draw electron configurations as well as find the. They are extremely small and are made up of even smaller particles. Scientists once thought the most fundamental building block of matter was a particle called the atom. Dramatic discoveries over the last century have completely changed our view of. The theories and discoveries of thousands of physicists since the 1930s have resulted in a remarkable insight into the fundamental structure of matter: These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. An element is a pure substance that is distinguished from all other matter by. The standard model of particle physics is scientists’ current best theory to describe the most basic building blocks of the universe. Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. State the fundamental building blocks of matter (i.e., quarks and leptons) and contrast them with the particles that were historically considered fundamental, and are the focus of science. Now we know that the atom is made of many smaller pieces, known as subatomic. In this live grade 10 physical sciences show we discuss the atom as the building block matter. These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. They are extremely small and are made up of even smaller particles. Everything in the universe is found. We consider the atomic theory as the foundation for understanding the interactions and changes in matter. All atoms consist of a small, positively charged. Additionally, we discuss isotopes and draw electron configurations as well as find the. Most normal matter is made up of atomic particles: The theories and discoveries of thousands of physicists since the 1930s have resulted in a remarkable insight into the fundamental structure of matter: Atoms are the basic building blocks that are used for every type of matter in the known universe. Additionally, we discuss isotopes and draw electron configurations as well as find the. Everything in the universe is found. We consider the atomic theory as the foundation for understanding the interactions and changes in matter. These basic building blocks lay the foundation. The theories and discoveries of thousands of physicists since the 1930s have resulted in a remarkable insight into the fundamental structure of matter: Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. In this live grade 10 physical sciences show we discuss the atom as the building block matter. Everything. Most normal matter is made up of atomic particles: Atoms are the basic building blocks that are used for every type of matter in the known universe. In this lesson we recognise that all matter is made up of atoms. All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. Additionally,. An element is a pure substance that is distinguished from all other matter by. Dramatic discoveries over the last century have completely changed our view of. In the past century, physicists have discovered new constituents of matter—quarks, gluons, neutrinos, and many others. These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. All. We consider the atomic theory as the foundation for understanding the interactions and changes in matter. Everything in the universe is found. Most normal matter is made up of atomic particles: It can exist as a gas, solid, liquid, or plasma of charged particles. We consider the atomic theory. State the fundamental building blocks of matter (i.e., quarks and leptons) and contrast them with the particles that were historically considered fundamental, and are the focus of science. The theories and discoveries of thousands of physicists since the 1930s have resulted in a remarkable insight into the fundamental structure of matter: Dramatic discoveries over the last century have completely changed. It can exist as a gas, solid, liquid, or plasma of charged particles. The theories and discoveries of thousands of physicists since the 1930s have resulted in a remarkable insight into the fundamental structure of matter: Scientists once thought the most fundamental building block of matter was a particle called the atom. Additionally, we discuss isotopes and draw electron configurations. Everything in the universe is found. In this live grade 10 physical sciences show we discuss the atom as the building block matter. An element is a pure substance that is distinguished from all other matter by. Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. Atoms are the basic. These basic building blocks have been linked together into a. They are extremely small and are made up of even smaller particles. In the past century, physicists have discovered new constituents of matter—quarks, gluons, neutrinos, and many others. All atoms consist of a small, positively charged. We consider the atomic theory as the foundation for understanding the interactions and changes. In this live grade 10 physical sciences show we discuss the atom as the building block matter. Atoms are the basic building blocks that are used for every type of matter in the known universe. Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. It explains how particles called quarks. It explains how particles called quarks (which make up. An element is a pure substance that is distinguished from all other matter by. All atoms consist of a small, positively charged. Most normal matter is made up of atomic particles: These basic building blocks have been linked together into a. Scientists once thought the most fundamental building block of matter was a particle called the atom. In this lesson we recognise that all matter is made up of atoms. In the past century, physicists have discovered new constituents of matter—quarks, gluons, neutrinos, and many others. The standard model of particle physics is scientists’ current best theory to describe the most basic building blocks of the universe. We consider the atomic theory as the foundation for understanding the interactions and changes in matter. Everything in the universe is found. We consider the atomic theory. State the fundamental building blocks of matter (i.e., quarks and leptons) and contrast them with the particles that were historically considered fundamental, and are the focus of science. They are extremely small and are made up of even smaller particles. The theories and discoveries of thousands of physicists since the 1930s have resulted in a remarkable insight into the fundamental structure of matter: Additionally, we discuss isotopes and draw electron configurations as well as find the.PPT Chemistry PowerPoint Presentation, free download ID9706406
Lesson 3.1 The Building Blocks of Matter YouTube
Basic Chemistry Section 2.1 (Matter). ppt download
PPT The Building Blocks of Matter Atoms PowerPoint Presentation
Define matter Tutorix
Building blocks of matter g3
PPT The Building Blocks of Matter Atoms PowerPoint Presentation
ATOMS Basic building blocks of all matter. ppt video online download
PPT I. What is an atom? PowerPoint Presentation, free download ID
PPT ATOMS The building blocks of matter PowerPoint Presentation, free
It Can Exist As A Gas, Solid, Liquid, Or Plasma Of Charged Particles.
These Basic Building Blocks Lay The Foundation For All Of The Ambitious Projects Detailed Throughout This Course.
All Matter In The Natural World Is Composed Of One Or More Of The 92 Fundamental Substances Called Elements.
Atoms Are The Basic Building Blocks That Are Used For Every Type Of Matter In The Known Universe.
Related Post: