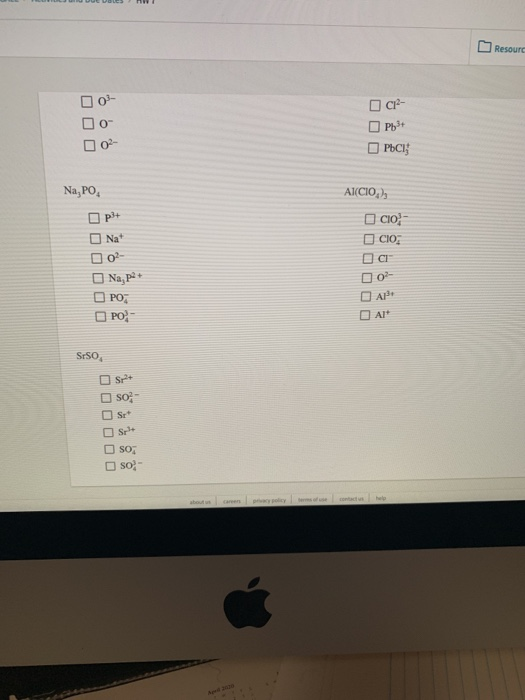
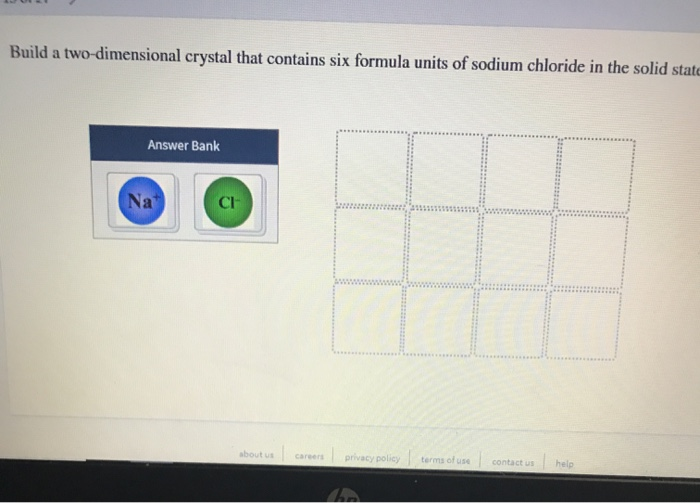
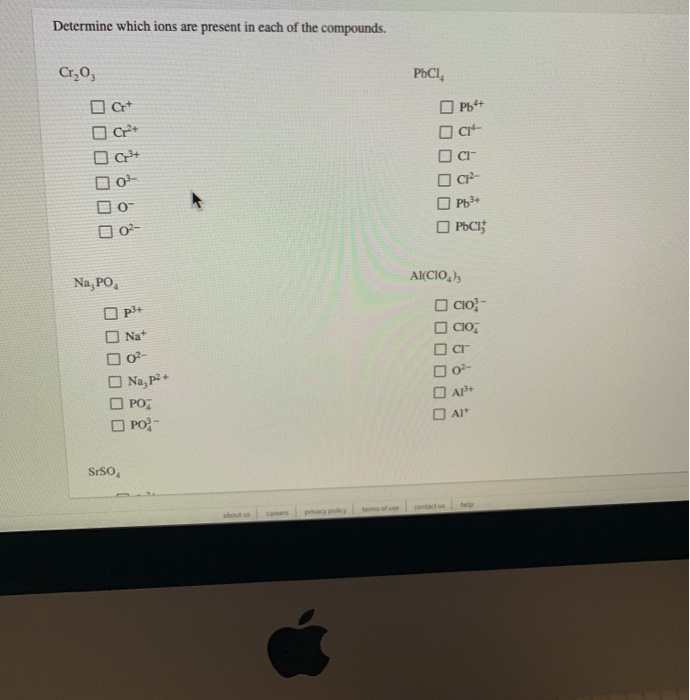
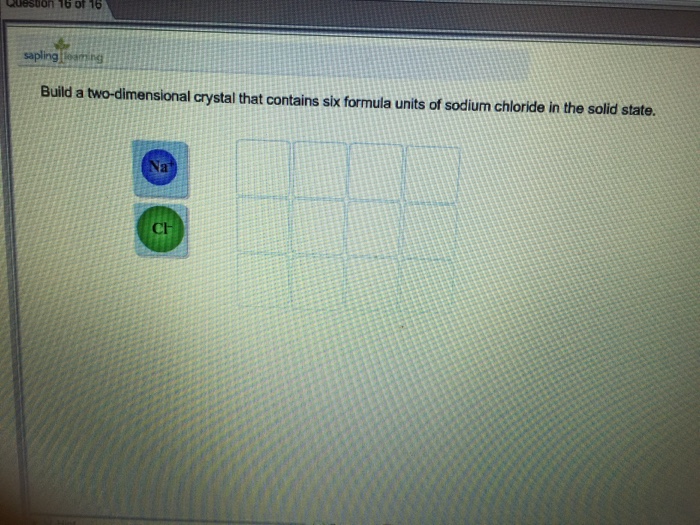
Build A Two Dimensional Crystal Of Sodium Chloride
Build A Two Dimensional Crystal Of Sodium Chloride - Ionic crystals formed from the ions. This demonstrates the 1:1 stoichiometry characteristic. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Our expert help has broken down your problem into. Your solution’s ready to go! Salt occurs in this structure as well. Now, na2cl and na3cl two. The unit cell of the crystal comprises. The cation is surrounded by a definite number of anions. Sodium chloride (nacl) forms a cubic crystal lattice. Your solution’s ready to go! This demonstrates the 1:1 stoichiometry characteristic. A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). Salt occurs in this structure as well. Sodium chloride has a face. The cation is surrounded by a definite number of anions. Sodium chloride (nacl) forms a cubic crystal lattice. Ionic crystals formed from the ions. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Now, na2cl and na3cl two. The cation is surrounded by a definite number of anions. Sodium chloride has a face. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Sodium chloride (nacl) forms a cubic crystal lattice. The unit cell of the crystal comprises. A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). Now, na2cl and na3cl two. Your solution’s ready to go! This demonstrates the 1:1 stoichiometry characteristic. Ionic crystals formed from the ions. This demonstrates the 1:1 stoichiometry characteristic. Ionic crystals formed from the ions. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Now, na2cl and na3cl two. Your solution’s ready to go! Your solution’s ready to go! Sodium chloride (nacl) forms a cubic crystal lattice. A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). The unit cell of the crystal comprises. Salt occurs in this structure as well. Your solution’s ready to go! The cation is surrounded by a definite number of anions. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: This demonstrates the 1:1 stoichiometry characteristic. Sodium chloride (nacl) forms a cubic crystal lattice. Our expert help has broken down your problem into. A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). This demonstrates the 1:1 stoichiometry characteristic. Now, na2cl and na3cl two. The cation is surrounded by a definite number of anions. The unit cell of the crystal comprises. Your solution’s ready to go! A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). Ionic crystals formed from the ions. Our expert help has broken down your problem into. Now, na2cl and na3cl two. The cation is surrounded by a definite number of anions. A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). Salt occurs in this structure as well. Sodium chloride has a face. The unit cell of the crystal comprises. Sodium chloride has a face. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Salt occurs in this structure as well. The cation is surrounded by a definite number of anions. This demonstrates the 1:1 stoichiometry characteristic. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Sodium chloride (nacl) forms a cubic crystal lattice. Our expert help has broken down your problem into. Your solution’s ready to go! The cation is surrounded by a definite number of anions. A crystal lattice is constructed by connecting adjacent equivalent points (lattice points). Your solution’s ready to go! The unit cell of the crystal comprises. Sodium chloride (nacl) forms a cubic crystal lattice. Now, na2cl and na3cl two. Build a two dimensional crystal that contains six formula units of sodium cholride in the solid… a: Our expert help has broken down your problem into. Ionic crystals formed from the ions.Solved Build a twodimensional crystal that contains six
SOLVED Build a twodimensional crystal that contains six formula units
Sodium Chloride Chemistry Crystal Structure Nacl 2d Model Clipart
Solved Build a twodimensional crystal that contains six
Draw The Molecular Structure Of Sodium Chloride Ananot1
Build a twodimensional crystal that contains six formula un Quizlet
Solved Build a twodimensional crystal that contains six
Atomic Structure Model Chemistry Sodium Chloride Crystal Demo Teaching
Solved builds a twodimensional crystal that contains six
Complete the model of a sodium chloride crystal by dragging the
Salt Occurs In This Structure As Well.
This Demonstrates The 1:1 Stoichiometry Characteristic.
Sodium Chloride Has A Face.
Related Post: