Building Up Principle
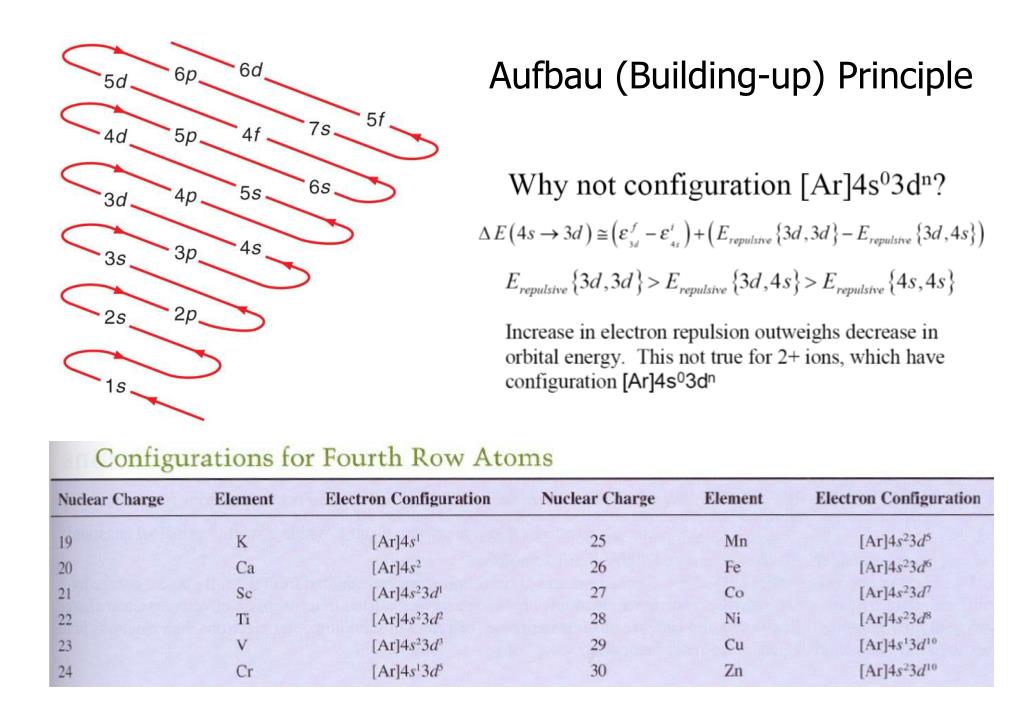
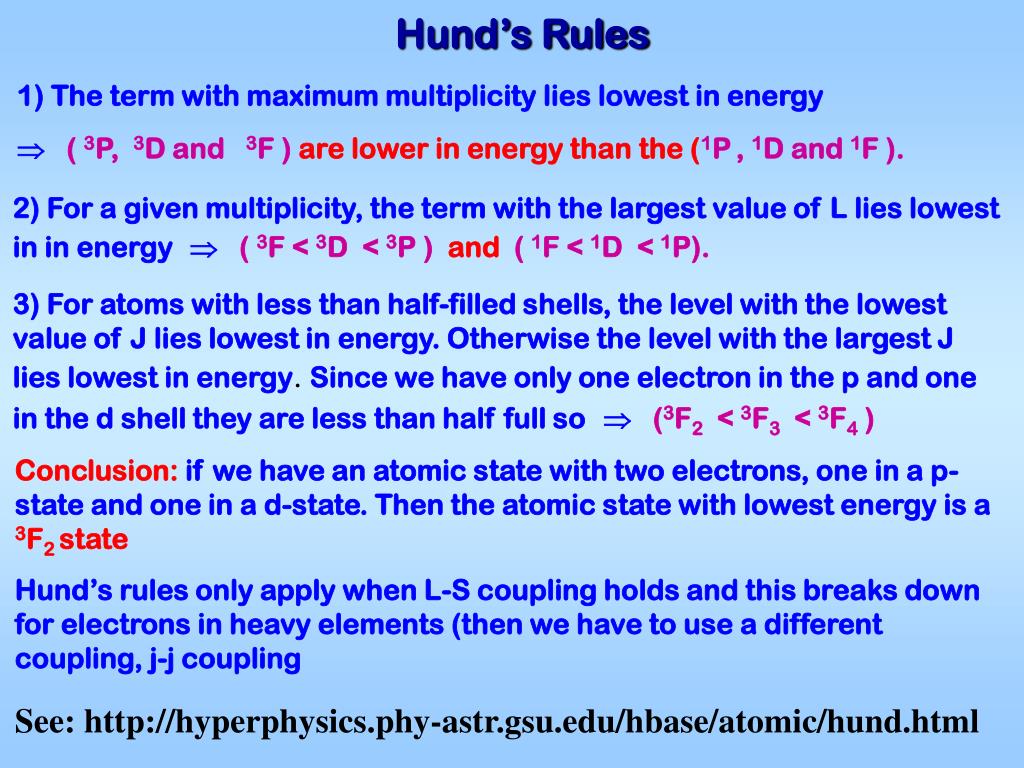
Building Up Principle - ‘aufbau’ is a german word that means construction or build up. Each added electron will enter the orbitals in the order of increasing energy; This principle is based on the idea that electrons strive to achieve the. N+l rule also known as madelung rule or klechkowski rule. The aufbau principle is based on the pauli exclusion principle and states that electrons occupy orbitals in a way that creates the lowest energy configuration, or the. In absence of external forces electrons in an atom fill lowest energy levels first. According to this rule, orbitals are filled based on the sum of the. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. These orbitals are filled in the order of n+l rule. The number of electrons in an atom is equal to the atomic number; The aufbau principle is based on the pauli exclusion principle and states that electrons occupy orbitals in a way that creates the lowest energy configuration, or the. Aufbau principle is principle of quantum mechanics. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. The aufbau (building up) principle: Key aspects of culture building. ‘aufbau’ is a german word that means construction or build up. The number of electrons in an atom is equal to the atomic number; The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. N+l rule also known as madelung rule or klechkowski rule. In absence of external forces electrons in an atom fill lowest energy levels first. Each added electron will enter the orbitals in the order of increasing energy; This principle is based on the idea that electrons strive to achieve the. These orbitals are filled in the order of n+l rule. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same. In absence of external forces electrons in an atom fill lowest energy levels first. The aufbau (building up) principle: These orbitals are filled in the order of n+l rule. ‘aufbau’ is a german word that means construction or build up. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom. This principle is based on the idea that electrons strive to achieve the. These orbitals are filled in the order of n+l rule. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. ‘aufbau’ is a german word that means construction or build up. According to this rule, orbitals are filled based on. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. This principle is based on the idea that electrons strive to achieve the. ‘aufbau’ is a german word that means construction or build up. Aufbau principle is principle of quantum mechanics. According to this rule, orbitals are filled based on the sum of. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. The aufbau principle is based on the pauli exclusion principle and states that electrons occupy orbitals in a way that creates the lowest energy configuration, or the. This principle is based on the idea that electrons strive to achieve the. Each added electron. Each added electron will enter the orbitals in the order of increasing energy; In absence of external forces electrons in an atom fill lowest energy levels first. N+l rule also known as madelung rule or klechkowski rule. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. These orbitals are filled in the. According to this rule, orbitals are filled based on the sum of the. These orbitals are filled in the order of n+l rule. The aufbau principle is based on the pauli exclusion principle and states that electrons occupy orbitals in a way that creates the lowest energy configuration, or the. This principle is based on the idea that electrons strive. The number of electrons in an atom is equal to the atomic number; These orbitals are filled in the order of n+l rule. Key aspects of culture building. N+l rule also known as madelung rule or klechkowski rule. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have. According to this rule, orbitals are filled based on the sum of the. ‘aufbau’ is a german word that means construction or build up. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. This principle is based on the idea that electrons strive to achieve the. The number of electrons in an. ‘aufbau’ is a german word that means construction or build up. According to this rule, orbitals are filled based on the sum of the. In absence of external forces electrons in an atom fill lowest energy levels first. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. The number of electrons in. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. The number of electrons in an atom is equal to the atomic number; N+l rule also known as madelung rule or klechkowski rule. Aufbau principle is principle of quantum mechanics. This principle is based on the idea that electrons strive to achieve the. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. Key aspects of culture building. ‘aufbau’ is a german word that means construction or build up. Each added electron will enter the orbitals in the order of increasing energy; The aufbau principle is based on the pauli exclusion principle and states that electrons occupy orbitals in a way that creates the lowest energy configuration, or the. These orbitals are filled in the order of n+l rule.PPT Lecture 9. ManyElectron Atoms PowerPoint Presentation, free
SOLUTION Aufbau principle or building up principle Studypool
PPT Atomic Physics PowerPoint Presentation, free download ID6221394
Atomic Structure. ppt download
“Building up” the atoms in the periodic table ppt download
PPT Chapter 9 Atomic Structure PowerPoint Presentation, free download
Electronic configuration
PPT Chapter 6 PowerPoint Presentation, free download ID6134347
Aufbau's building up principle Ch.1 (atomic structure) Chemistry
Building up principle chemistry 6A CHEM 6A Studocu
The Aufbau (Building Up) Principle:
In Absence Of External Forces Electrons In An Atom Fill Lowest Energy Levels First.
According To This Rule, Orbitals Are Filled Based On The Sum Of The.
Related Post:

..jpg)





