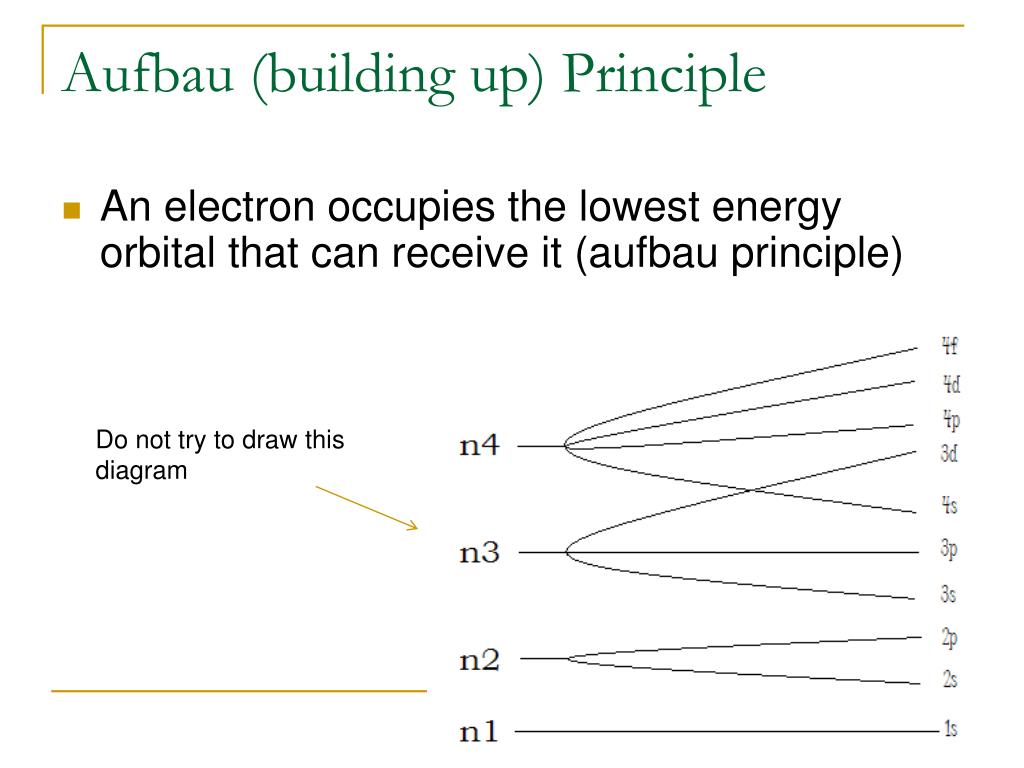
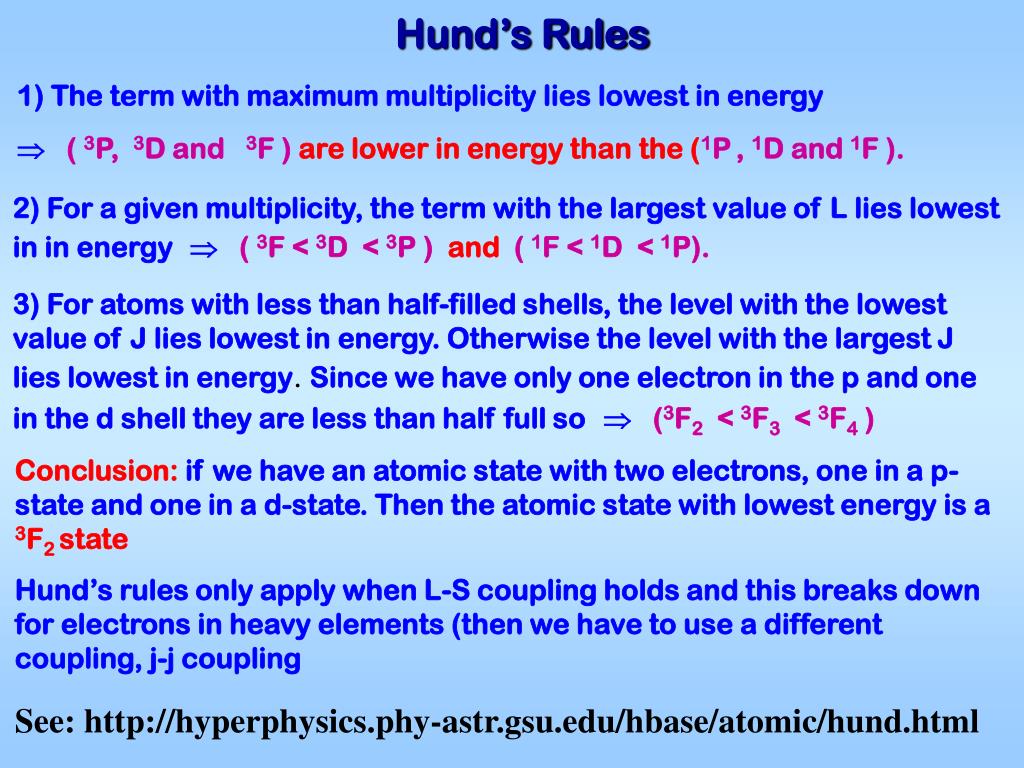
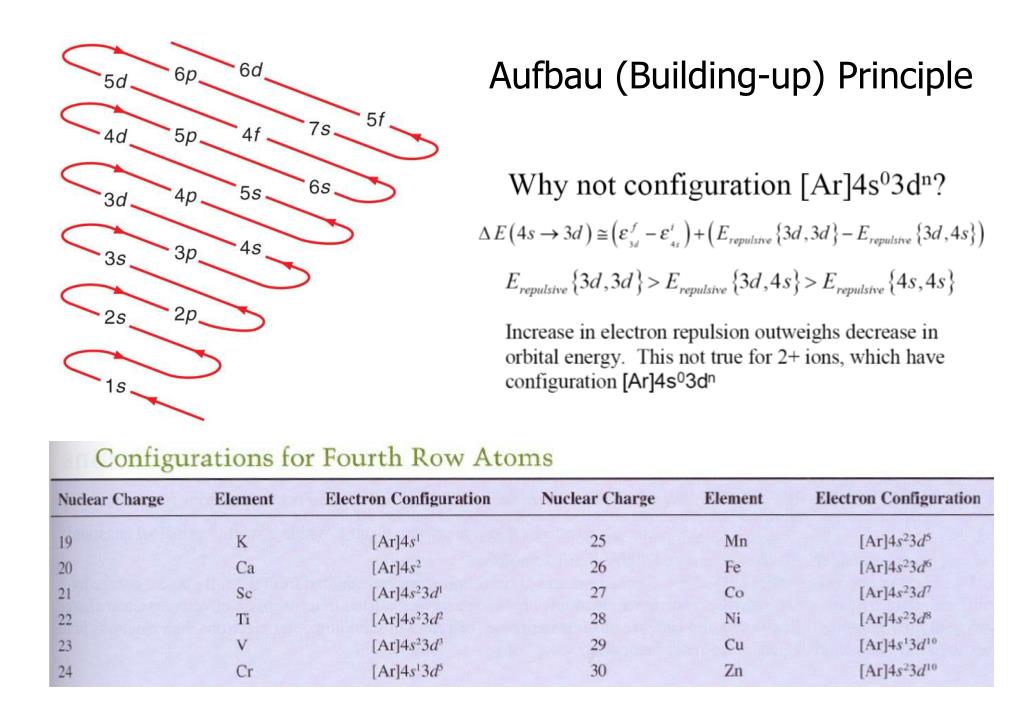
Buildingup Principle
Buildingup Principle - Proposed by the danish physicist niels bohr in the early. Aufbau (german aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. In atomic physics and quantum chemistry, the aufbau principle , also called the aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available energy, then fill subshells of higher energy. Talk to real customers before writing code. It is worth noting that in reality atoms are not built by adding. In this way, the electrons of an atom or ion form the most stable electron configuration poss… Sometimes, the hardest lessons are the ones we have to learn more. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. For example, the 1s subshell is filled before the 2s subshell is occupied. The building up (aufbau principle) states that an electron occupies orbitals in order from lowest to highest energy. According to this rule, orbitals are filled based on the sum of the. Sometimes, the hardest lessons are the ones we have to learn more. Talk to real customers before writing code. B y observing their selection rules, splitting in external fields etc., the characteristics o f. Proposed by the danish physicist niels bohr. The lowest energy sublevel is the 1s sublevel, then as we go on. In this way, the electrons of an atom or ion form the most stable electron configuration poss… The three core principles of getting out of the building 1. The aufbau principle determines an atom's electron configuration by adding electrons to atomic orbitals following a defined set of rules. In atomic physics and quantum chemistry, the aufbau principle , also called the aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available energy, then fill subshells of higher energy. In this way, the electrons of an atom or ion form the most stable electron configuration poss… The building up (aufbau principle) states that an electron occupies orbitals in order from lowest to highest energy. It is worth noting that in reality atoms are not built by adding. The three core principles of getting out of the building 1. B. Sometimes, the hardest lessons are the ones we have to learn more. It is worth noting that in reality atoms are not built by adding. Aufbau (german aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. Remember, the main energy level is given by the principal. The aufbau principle. The lowest energy sublevel is the 1s sublevel, then as we go on. Aufbau (german aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. Remember, the main energy level is given by the principal. The three core principles of getting out of the building 1. It is worth noting. The building up (aufbau principle) states that an electron occupies orbitals in order from lowest to highest energy. Sometimes, the hardest lessons are the ones we have to learn more. The aufbau principle determines an atom's electron configuration by adding electrons to atomic orbitals following a defined set of rules. The lowest energy sublevel is the 1s sublevel, then as. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. The aufbau principle determines an atom's electron configuration by adding electrons to atomic orbitals following a defined set of rules. In this way, the electrons of an atom or ion form the. Aufbau (german aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. According to this rule, orbitals are filled based on the sum of the. Sometimes, the hardest lessons are the ones we have to learn more. Proposed by the danish physicist niels bohr. Remember, the main energy level is. Remember, the main energy level is given by the principal. According to this rule, orbitals are filled based on the sum of the. The aufbau principle determines an atom's electron configuration by adding electrons to atomic orbitals following a defined set of rules. It is worth noting that in reality atoms are not built by adding. The lowest energy sublevel. Proposed by the danish physicist niels bohr in the early. The three core principles of getting out of the building 1. For example, the 1s subshell is filled before the 2s subshell is occupied. Remember, the main energy level is given by the principal. Proposed by the danish physicist niels bohr. The building up (aufbau principle) states that an electron occupies orbitals in order from lowest to highest energy. B y observing their selection rules, splitting in external fields etc., the characteristics o f. Talk to real customers before writing code. Proposed by the danish physicist niels bohr in the early. Aufbau (german aufbauen, “to build up”) principle tells us that. According to this rule, orbitals are filled based on the sum of the. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. The three core principles of getting out of the building 1. In this way, the electrons of an atom. In atomic physics and quantum chemistry, the aufbau principle , also called the aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available energy, then fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration poss… Proposed by the danish physicist niels bohr. The three core principles of getting out of the building 1. Proposed by the danish physicist niels bohr in the early. Aufbau (german aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. Talk to real customers before writing code. The lowest energy sublevel is the 1s sublevel, then as we go on. Sometimes, the hardest lessons are the ones we have to learn more. The aufbau principle originates from the pauli’s exclusion principle which says that no two fermions (e.g., electrons) in an atom can have the same set of quantum numbers,. Remember, the main energy level is given by the principal. According to this rule, orbitals are filled based on the sum of the.PPT Chapter 6 PowerPoint Presentation, free download ID6134347
Aufbau Principle Periodic Table
SOLUTION Aufbau principle or building up principle Studypool
“Building up” the atoms in the periodic table ppt download
PPT Chapter 9 Atomic Structure PowerPoint Presentation, free download
PPT Atomic Physics PowerPoint Presentation, free download ID6221394
Explain Aufbau Principle?? Fullonstudy
PPT Lecture 9. ManyElectron Atoms PowerPoint Presentation, free
Aufbau's building up principle Ch.1 (atomic structure) Chemistry
Electronic configuration
The Building Up (Aufbau Principle) States That An Electron Occupies Orbitals In Order From Lowest To Highest Energy.
It Is Worth Noting That In Reality Atoms Are Not Built By Adding.
B Y Observing Their Selection Rules, Splitting In External Fields Etc., The Characteristics O F.
The Aufbau Principle Determines An Atom's Electron Configuration By Adding Electrons To Atomic Orbitals Following A Defined Set Of Rules.
Related Post: