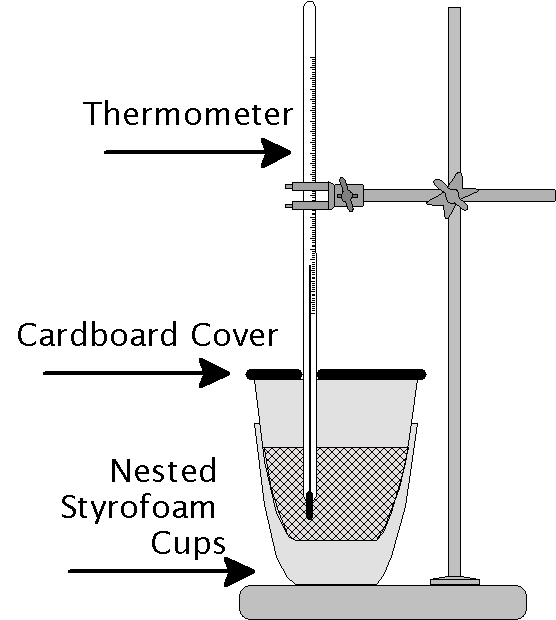
How To Build A Calorimeter
How To Build A Calorimeter - These calories are not the same as those used to refer to. Here, we talk about what it takes to make a calorimeter, the lab apparatus used to run a calorimetry experiment. Calorimeters are used to measure potential energy. It usually consists of two styrofoam. To make a simple calorimeter, you will need a container to hold water, a thermometer to measure temperature changes, and a heat source to generate heat. A calorie is the energy it takes to heat 1 ml of water 1 degree celsius. Here's a short video showing you how to make a calorimeter for nc science olympiad (or whatever you want to make yours for)! A coffee cup calorimeter is a simple device used to measure the heat of chemical reactions or physical changes as well as heat capacity. Measure the mass of the empty calorimeter with a balance. In your groups, plan how to build a calorimeter out of the provided materials. Calorimeters are used to measure potential energy. In this article, we will discuss how to identify the necessary parts to build a calorimeter, provide two versions of the recipe for building one, highlight four interesting trends related to. A calorie is the energy it takes to heat 1 ml of water 1 degree celsius. These calories are not the same as those used to refer to food on nutrition labels, dietary plans, etc., which are known as calories or kcal (1000 normal calories). Record on a data table. Measure the mass of the empty calorimeter with a balance. To make a simple calorimeter, you will need a container to hold water, a thermometer to measure temperature changes, and a heat source to generate heat. These calories are not the same as those used to refer to. Make sure the water is not hot enough to cause any damage to your thermometer’s container. I will show you how to make diy calorimeter and home version of 'leslie cube'. In your groups, plan how to build a calorimeter out of the provided materials. To make a simple calorimeter, you will need a container to hold water, a thermometer to measure temperature changes, and a heat source to generate heat. Here, we talk about what it takes to make a calorimeter, the lab apparatus used to run a calorimetry experiment.. Calorimeters are used to measure potential energy. Make sure the water is not hot enough to cause any damage to your thermometer’s container. Brainstorm ideas about how you can burn the food to release its energy, how you will capture the released. Both pieces of equipment are necessary to complete gcse practicals in uk, but even if you from different.. It usually consists of two styrofoam. On the other hand, placing the thermometer in ice water will demonstrate contraction, as the liquid. Insulated container (such as a polystyrene or plastic drinking cup) 2. Here, we talk about what it takes to make a calorimeter, the lab apparatus used to run a calorimetry experiment. Calorimeters are used to measure potential energy. Calorimeters are used to measure potential energy. With some simple, everyday materials,. Record on a data table. On the other hand, placing the thermometer in ice water will demonstrate contraction, as the liquid. Insulated container (such as a polystyrene or plastic drinking cup) 2. Insulated container (such as a polystyrene or plastic drinking cup) 2. A coffee cup calorimeter is a simple device used to measure the heat of chemical reactions or physical changes as well as heat capacity. These calories are not the same as those used to refer to food on nutrition labels, dietary plans, etc., which are known as calories or. Calorimeters are used to measure potential energy. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. On the other hand, placing the thermometer in ice water will demonstrate contraction, as the liquid. It usually consists of two styrofoam. I will. Make sure the water is not hot enough to cause any damage to your thermometer’s container. In your groups, plan how to build a calorimeter out of the provided materials. With some simple, everyday materials,. On the other hand, placing the thermometer in ice water will demonstrate contraction, as the liquid. A calorimeter is an object used for calorimetry, or. Here's a short video showing you how to make a calorimeter for nc science olympiad (or whatever you want to make yours for)! Calorimeters are used to measure potential energy. These calories are not the same as those used to refer to. On the other hand, placing the thermometer in ice water will demonstrate contraction, as the liquid. Make sure. On the other hand, placing the thermometer in ice water will demonstrate contraction, as the liquid. With some simple, everyday materials,. Record on a data table. Here's a short video showing you how to make a calorimeter for nc science olympiad (or whatever you want to make yours for)! Brainstorm ideas about how you can burn the food to release. These calories are not the same as those used to refer to food on nutrition labels, dietary plans, etc., which are known as calories or kcal (1000 normal calories). A calorie is the energy it takes to heat 1 ml of water 1 degree celsius. Make sure the water is not hot enough to cause any damage to your thermometer’s. Calorimeters are used to measure potential energy. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. In your groups, plan how to build a calorimeter out of the provided materials. A calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. In this article, we will discuss how to identify the necessary parts to build a calorimeter, provide two versions of the recipe for building one, highlight four interesting trends related to. Insulated container (such as a polystyrene or plastic drinking cup) 2. To build a simple calorimeter, you will need a container to hold water, a thermometer to measure temperature changes, and a heat source to generate heat. These calories are not the same as those used to refer to food on nutrition labels, dietary plans, etc., which are known as calories or kcal (1000 normal calories). Make sure the water is not hot enough to cause any damage to your thermometer’s container. It usually consists of two styrofoam. A calorie is the energy it takes to heat 1 ml of water 1 degree celsius. Both pieces of equipment are necessary to complete gcse practicals in uk, but even if you from different. These calories are not the same as those used to refer to. Calorimeters are used to measure potential energy. In this article, we will show you how to create a simple yet effective diy calorimeter. Record on a data table.How to Build a Calorimeter (with Pictures) wikiHow
How To Build A Calorimeter
How To Set Up Calorimeter at Paul Brose blog
How to Build a Calorimeter 12 Steps (with Pictures) wikiHow
How to Build a Calorimeter (with Pictures) wikiHow
Constructing a Calorimeter YouTube
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and
How to Build a Calorimeter (with Pictures) wikiHow
How To Build A Calorimeter
How to Build a Calorimeter (with Pictures) wikiHow
With Some Simple, Everyday Materials,.
A Calorie Is The Energy It Takes To Heat 1 Ml Of Water 1 Degree Celsius.
Measure The Mass Of The Empty Calorimeter With A Balance.
Here, We Talk About What It Takes To Make A Calorimeter, The Lab Apparatus Used To Run A Calorimetry Experiment.
Related Post: