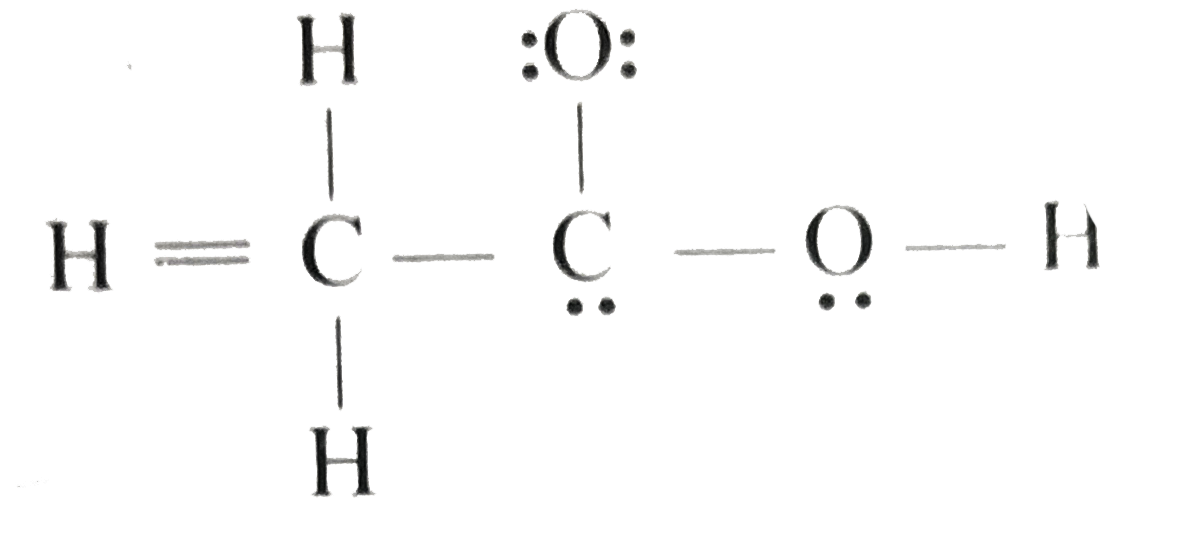
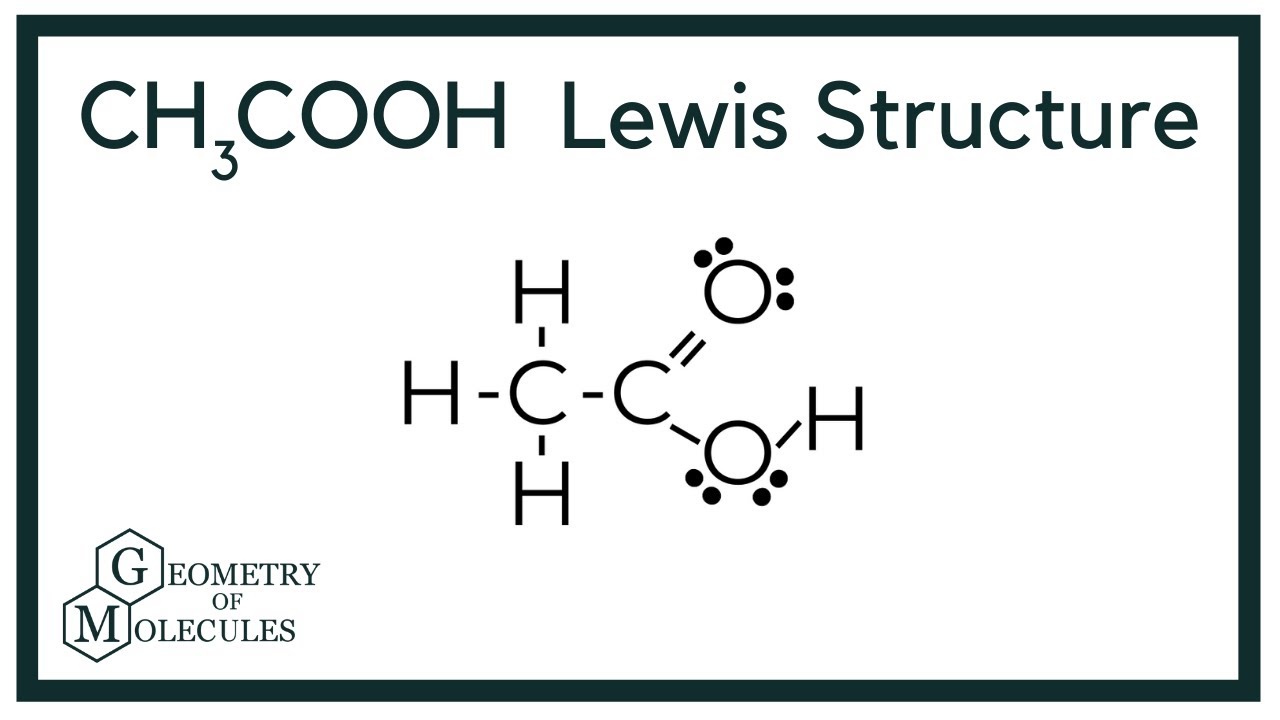
Hwo To Know How To Build Acetic Acid Lewis Structure
Hwo To Know How To Build Acetic Acid Lewis Structure - In h 2 o, for example, there is a bonding pair of electrons between oxygen and. By considering valence electrons, the octet rule, lone pairs, and the role of hydrogen atoms, we can accurately build the lewis structure and gain valuable insights into. Find the total valence electrons for the ch3cooh molecule. It's actually a pretty common functional group in. Be certain you include any lone pairs. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Let's draw the acetic acid lewis structure. Unlock the secrets of chemical bonding with the lewis structure of acetic acid. Draw the lewis structure for acetic acid (c2h4o2). To draw the lewis structure of acetic acid (ch₃cooh), we need to determine the number of valence electrons in each atom and distribute them around the central carbon. “how to build acetic acid lewis structure” delves into the process of representing the covalent bonding and electron pairs in acetic acid using lewis structure notation. Draw the lewis structure for acetic acid (c2h4o2). Be certain you include any lone pairs. Learn about the lewis dot structure for acetic acid (ch3cooh) and other properties of this compound! In h 2 o, for example, there is a bonding pair of electrons between oxygen and. In this article, we’ll break down the acetic acid structure in simple terms, allowing you to grasp its importance effortlessly. The key to drawing this structure is realizing this cooh right here is what we call a functional group. By considering valence electrons, the octet rule, lone pairs, and the role of hydrogen atoms, we can accurately build the lewis structure and gain valuable insights into. Find the total valence electrons for the ch3cooh molecule. Acetic acid, also known as. Put the least electronegative atom in. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Acetic acid molecules have one hydroxy group and one carbonyl group. I also go over hybridization, shape, sigma, pi bonding and bond angles. In this article, we’ll break down the acetic acid structure in simple terms, allowing. Acetic acid, also known as. There are two many electrons in the sketch 24 electrons are required, but only 22 are shown one of the o's. It's actually a pretty common functional group in. In h 2 o, for example, there is a bonding pair of electrons between oxygen and. Learn about the lewis dot structure for acetic acid (ch3cooh). To draw the lewis structure of acetic acid (ch₃cooh), we need to determine the number of valence electrons in each atom and distribute them around the central carbon. Find the total valence electrons for the ch3cooh molecule. The key to drawing this structure is realizing this cooh right here is what we call a functional group. Explore its molecular geometry,. Explain why this is not a good lewis structure. Acetic acid molecules have one hydroxy group and one carbonyl group. Draw the lewis structure for acetic acid (c2h4o2). I also go over hybridization, shape, sigma, pi bonding and bond angles. It's actually a pretty common functional group in. I quickly take you through how to draw the lewis structure of ch3cooh (acetic acid). Explore its molecular geometry, hybridization, polarity, and more in this comprehensive guide! Acetic acid, also known as. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. It's actually a pretty common functional group in. Learn about the lewis dot structure for acetic acid (ch3cooh) and other properties of this compound! Acetic acid molecules have one hydroxy group and one carbonyl group. Acetic acid, also known as. It's actually a pretty common functional group in. Find the total valence electrons for the ch3cooh molecule. Acetic acid molecules have one hydroxy group and one carbonyl group. By considering valence electrons, the octet rule, lone pairs, and the role of hydrogen atoms, we can accurately build the lewis structure and gain valuable insights into. There are two many electrons in the sketch 24 electrons are required, but only 22 are shown one of the o's. I. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Learn about the lewis dot structure for acetic acid (ch3cooh) and other properties of this compound! In this article, we’ll break down the acetic acid structure in simple terms, allowing you to grasp its importance effortlessly. Explore its molecular geometry, hybridization, polarity, and. Find the total valence electrons for the ch3cooh molecule. To draw the lewis structure of acetic acid (ch₃cooh), we need to determine the number of valence electrons in each atom and distribute them around the central carbon. There are two many electrons in the sketch 24 electrons are required, but only 22 are shown one of the o's. I quickly. In h 2 o, for example, there is a bonding pair of electrons between oxygen and. Be certain you include any lone pairs. To draw the lewis structure of acetic acid (ch₃cooh), we need to determine the number of valence electrons in each atom and distribute them around the central carbon. The acetic acid lewis structure should look like this:. I quickly take you through how to draw the lewis structure of ch3cooh (acetic acid). Learn about the lewis dot structure for acetic acid (ch3cooh) and other properties of this compound! The key to drawing this structure is realizing this cooh right here is what we call a functional group. Put the least electronegative atom in. Acetic acid, also known as. In this article, we’ll break down the acetic acid structure in simple terms, allowing you to grasp its importance effortlessly. Unlock the secrets of chemical bonding with the lewis structure of acetic acid. Acetic acid molecules have one hydroxy group and one carbonyl group. Let's draw the acetic acid lewis structure. The acetic acid lewis structure should look like this: To draw the lewis structure of acetic acid (ch₃cooh), we need to determine the number of valence electrons in each atom and distribute them around the central carbon. Be certain you include any lone pairs. In h 2 o, for example, there is a bonding pair of electrons between oxygen and. It's actually a pretty common functional group in. Explore its molecular geometry, hybridization, polarity, and more in this comprehensive guide! “how to build acetic acid lewis structure” delves into the process of representing the covalent bonding and electron pairs in acetic acid using lewis structure notation.Draw the Lewis structure for acetic acid. Quizlet
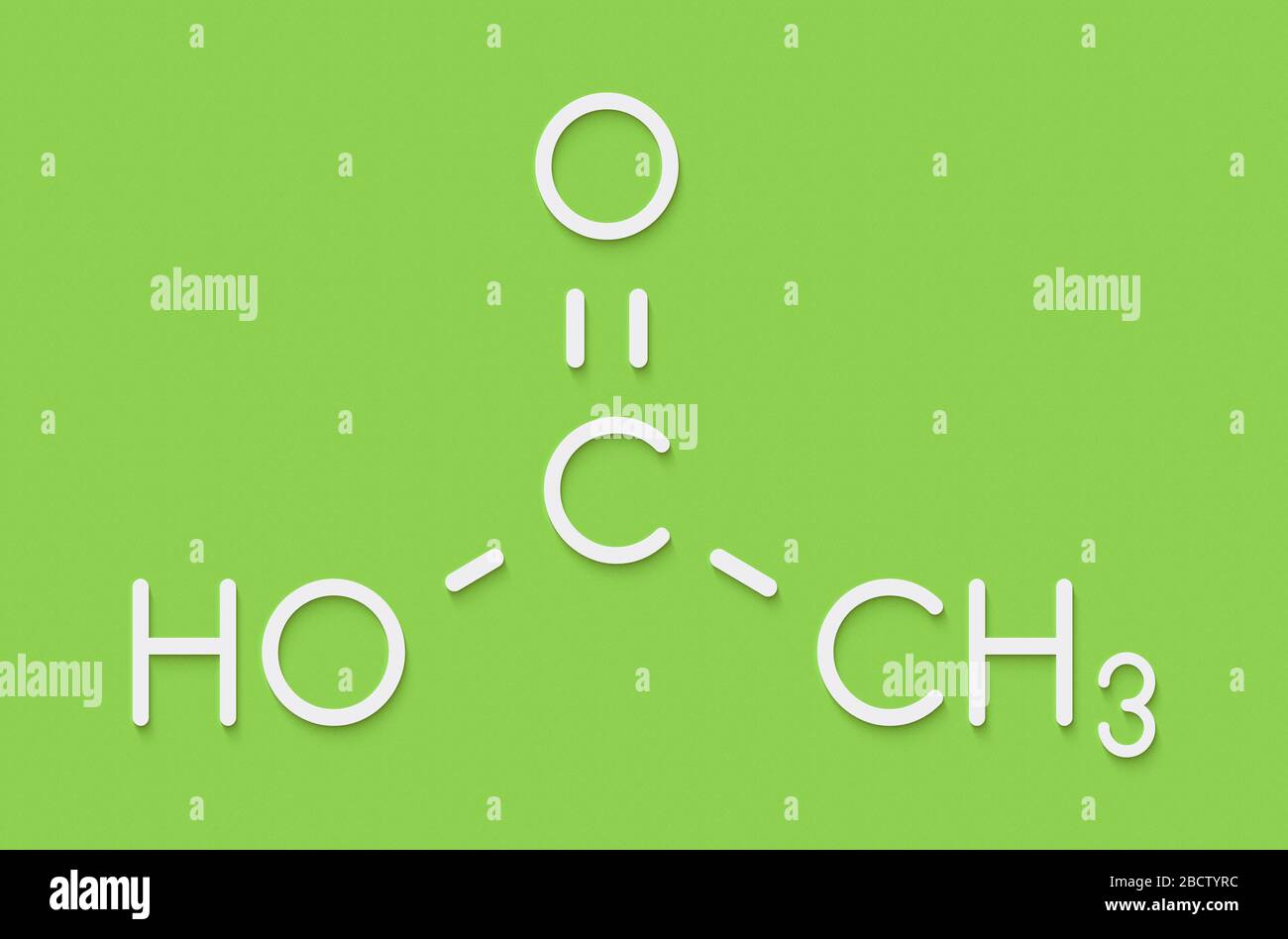
Lewis Structure Of Acetic Acid
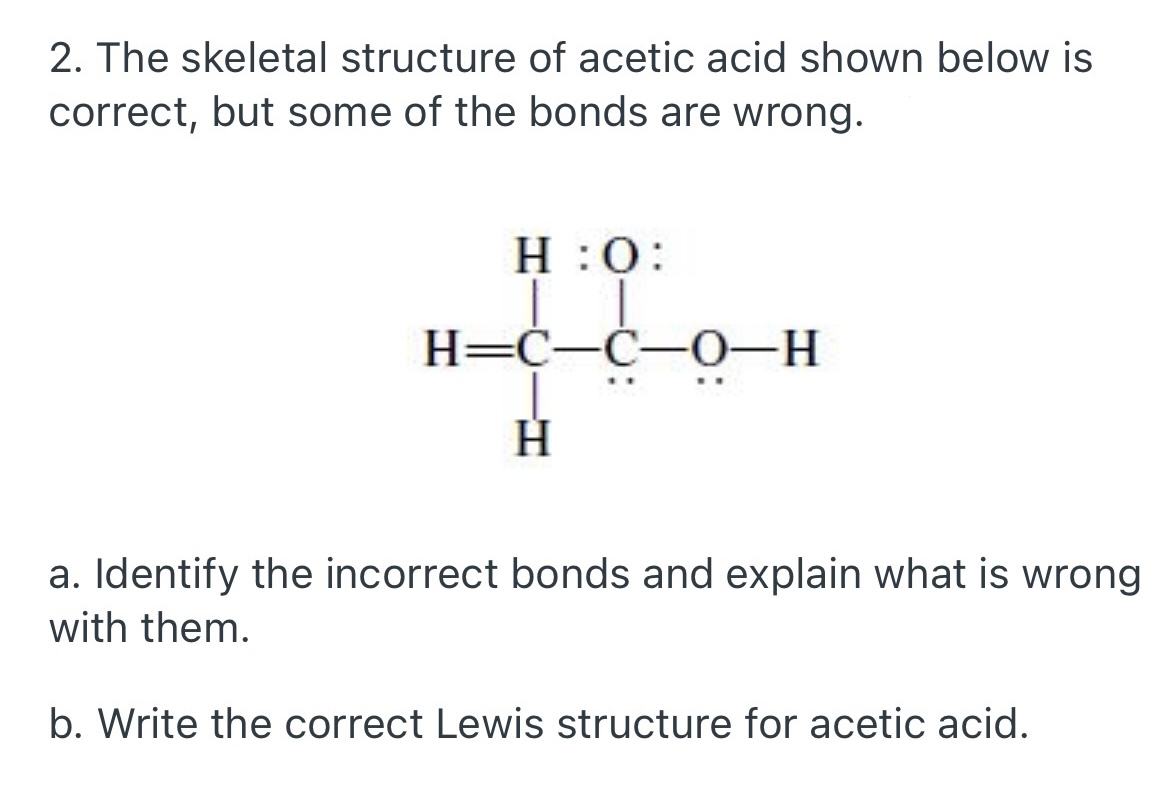
SOLVED The Lewis Structure For Acetic Acid (CH3COOH) Shown
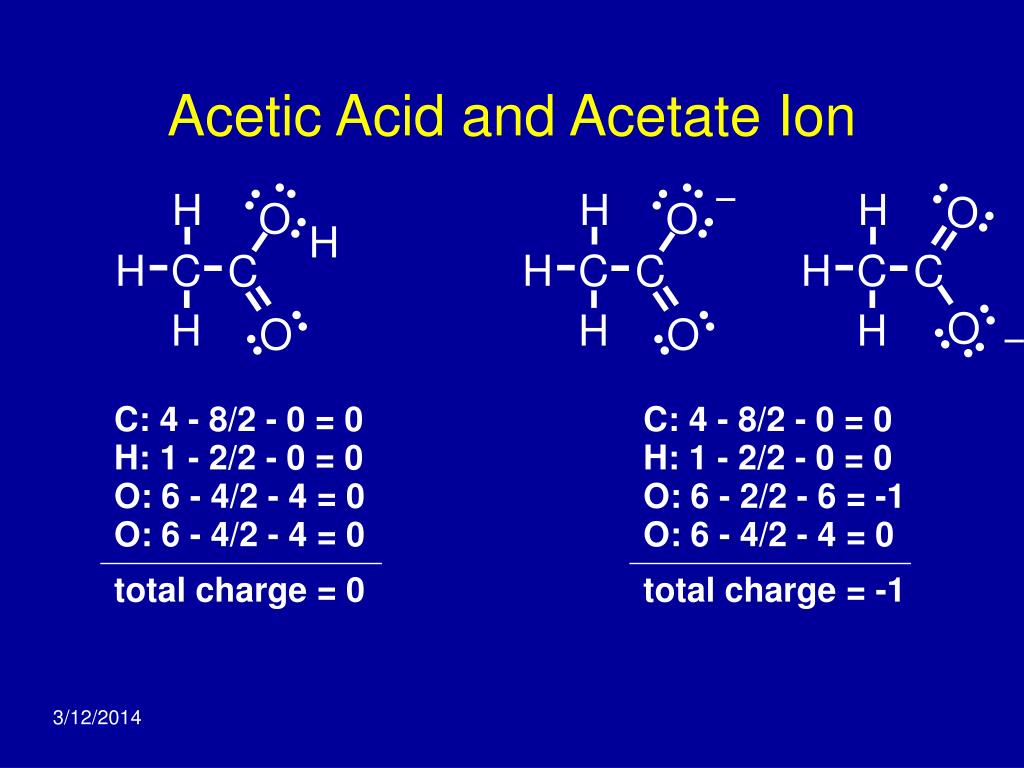
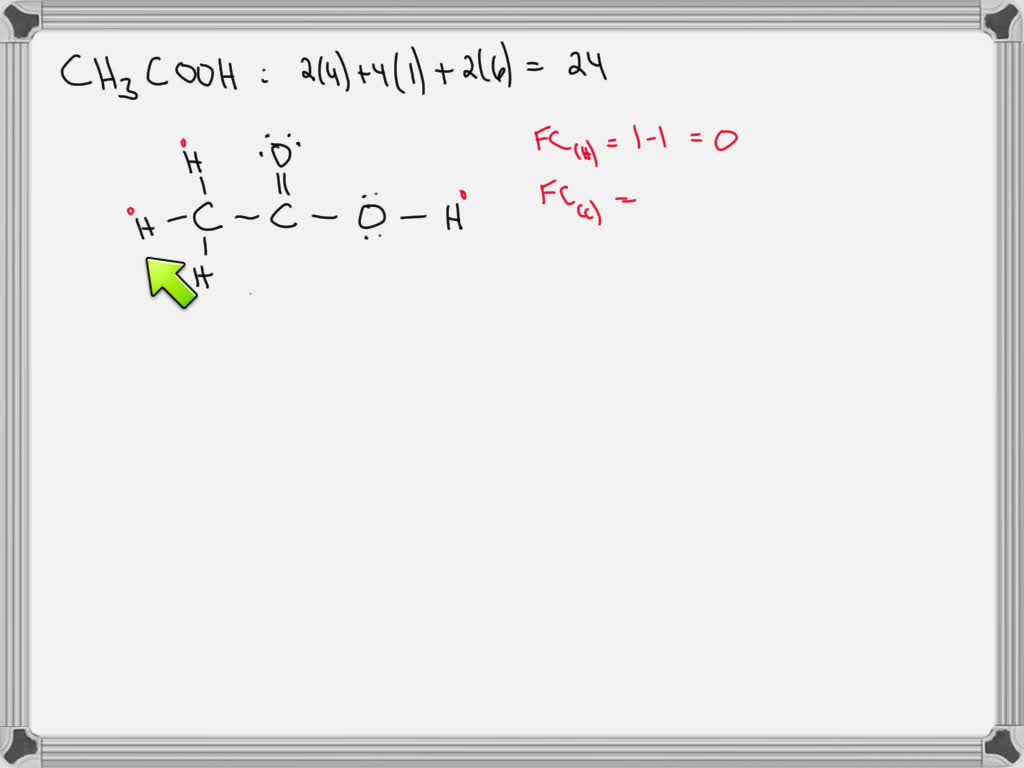
Acetic Acid Lewis Structure With Formal Charges
Acetic Acid Lewis Structure With Formal Charges
Acetic Acid Lewis Structure With Formal Charges
Acetic Acid Lewis Structure With Formal Charges
Lewis Dot Structure Of Acetic Acid
CH3COOH Lewis Structure (Acetic acid) YouTube
Acetic Acid Lewis Structure Resonance
Place A Bonding Pair Of Electrons Between Each Pair Of Adjacent Atoms To Give A Single Bond.
Explain Why This Is Not A Good Lewis Structure.
There Are Two Many Electrons In The Sketch 24 Electrons Are Required, But Only 22 Are Shown One Of The O's.
By Considering Valence Electrons, The Octet Rule, Lone Pairs, And The Role Of Hydrogen Atoms, We Can Accurately Build The Lewis Structure And Gain Valuable Insights Into.
Related Post: