Identify The Necessary Parts To Build A Calorimeter
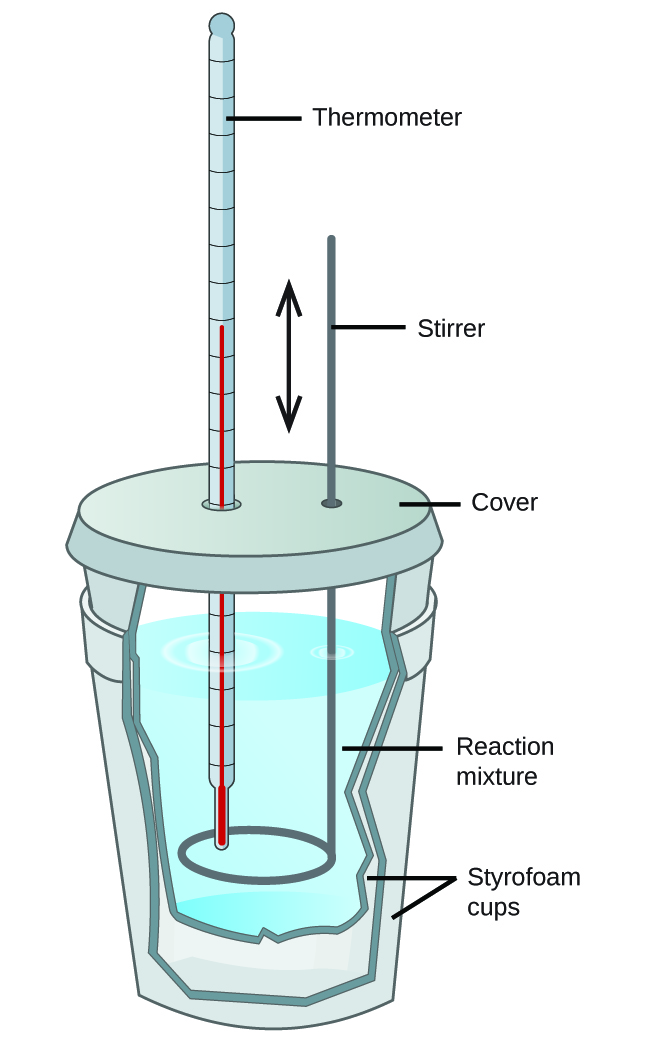
Identify The Necessary Parts To Build A Calorimeter - The necessary parts to build a calorimeter include an insulated container, thermometer, stirrer, calorimeter bomb (if necessary), and water as a heat sink. Learn how to create a simple diy calorimeter with materials you can find at home. #### variables and formulas a calorimeter. E) suppose you heat a metal object with a mass of 33.7 g to 95.1 oc and transfer it to a calorimeter containing 100.0 g of water at 18.7 oc. The law of conservation of energy states that ___________________. A cover helps to minimize heat exchange between the calorimeter and the surrounding. To build a simple calorimeter, you will need several key components: To build a calorimeter, the necessary parts typica. Not the question you’re looking for? The water and metal reach a final temperature of 22.4. A calorimeter is a device that measures the heat produced or absorbed during chemical reactions. Identify the necessary parts to build a calorimeter. Identify the necessary parts to build a calorimeter. To build a calorimeter, the necessary parts typica. Identify the necessary parts to build a calorimeter. Suppose you heat a metal object with a mass of 31.8 g to 95.7 °c and transfer it to a calorimeter containing 100.0 g of water at 18.9 °c. Not the question you’re looking for? What will be the final temperature of the mixed water, in oc? This calorimeter consists of a beaker, or instead, a container of anime material. Learn how to create a simple diy calorimeter with materials you can find at home. Identify the necessary parts to build a calorimeter. The necessary parts to build a calorimeter include an insulated container, thermometer, stirrer, calorimeter bomb (if necessary), and water as a heat sink. Learn how to create a simple diy calorimeter with materials you can find at home. To build a simple calorimeter, you will need several key components: Suppose you heat. A cover helps to minimize heat exchange between the calorimeter and the surrounding. #### variables and formulas a calorimeter. E) suppose you heat a metal object with a mass of 33.7 g to 95.1 oc and transfer it to a calorimeter containing 100.0 g of water at 18.7 oc. The necessary parts to build a calorimeter include an insulated container,. Identify the necessary parts to build a calorimeter. The water and metal reach a final. A calorimeter is a device used to experimentally determine the heat associated. #### variables and formulas a calorimeter. A calorimeter is a device that measures the heat produced or absorbed during chemical reactions. To determine which parts are necessary to build a calorimeter, let's first recall what is the calorimeter. The water and metal reach a final temperature of 22.4. The water and metal reach a final. A calorimeter is a device used to experimentally determine the heat associated. There are 3 steps to solve this one. The water and metal reach a final. This calorimeter consists of a beaker, or instead, a container of anime material. Learn how to create a simple diy calorimeter with materials you can find at home. Identify the necessary parts to build a calorimeter. Two versions of the recipe are provided for. A cover helps to minimize heat exchange between the calorimeter and the surrounding. Not the question you’re looking for? To determine which parts are necessary to build a calorimeter, let's first recall what is the calorimeter. There are 3 steps to solve this one. Two versions of the recipe are provided for. An exterior styrofoam cup an interior styrofoam cup an interior paper cup an exterior. In summary, building a calorimeter requires identifying the necessary parts such as a container, thermometer, insulating material, stirrer, and lid. Identify the necessary parts to build a calorimeter. A calorimeter is a device that measures the heat produced or absorbed during chemical reactions. Not the question. Here’s the best way to solve it. There are 3 steps to solve this one. Identify the necessary parts to build a calorimeter. Identify the necessary parts to build a calorimeter. The water in a calorimeter absorbing heat given off by a reaction or hot object. Here’s the best way to solve it. The water and metal reach a final temperature of 22.4. An exterior styrofoam cup an interior styrofoam cup an interior paper cup an exterior. What will be the final temperature of the mixed water, in oc? Here’s the best way to solve it. This calorimeter consists of a beaker, or instead, a container of anime material. Suppose you heat a metal object with a mass of 31.8 g to 95.7 °c and transfer it to a calorimeter containing 100.0 g of water at 18.9 °c. A calorimeter is a device that measures the heat produced or absorbed during chemical reactions. Learn how to. A calorimeter is a device that measures the heat produced or absorbed during chemical reactions. Identify the necessary parts to build a calorimeter. The water and metal reach a final temperature of 22.4. #### variables and formulas a calorimeter. A calorimeter is a device used to experimentally determine the heat associated. Here’s the best way to solve it. Identify the necessary parts to build a calorimeter. E) suppose you heat a metal object with a mass of 33.7 g to 95.1 oc and transfer it to a calorimeter containing 100.0 g of water at 18.7 oc. Identify the necessary parts to build a calorimeter. Here’s the best way to solve it. Suppose you heat a metal object with a mass of 31.8 g to 95.7 °c and transfer it to a calorimeter containing 100.0 g of water at 18.9 °c. The law of conservation of energy states that ___________________. Learn how to create a simple diy calorimeter with materials you can find at home. This calorimeter consists of a beaker, or instead, a container of anime material. Identify the necessary parts to build a calorimeter. Identify the necessary parts to build a calorimeter.Calorimeter Diagram
5.2 Calorimetry Chemistry
Calorimetry Chemistry Atoms First
Calorimeter And The Function at Judith Stanton blog
Calorimetry Grade12UChemistry
Calorimeter Definition, Uses, Diagram, & Facts Britannica
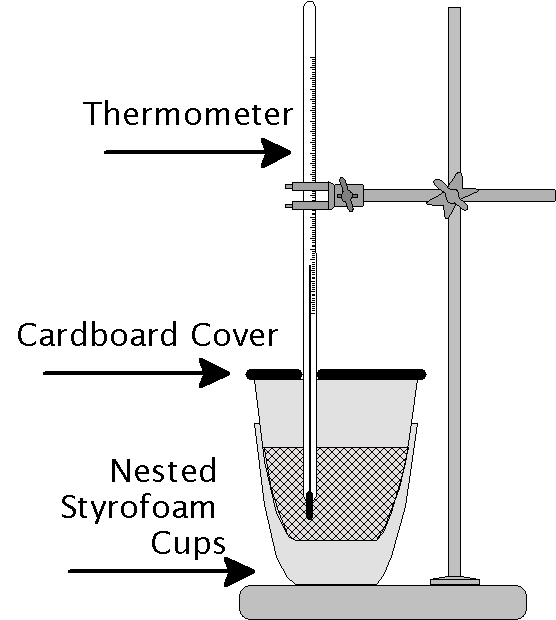
How to Build a Calorimeter (with Pictures) wikiHow
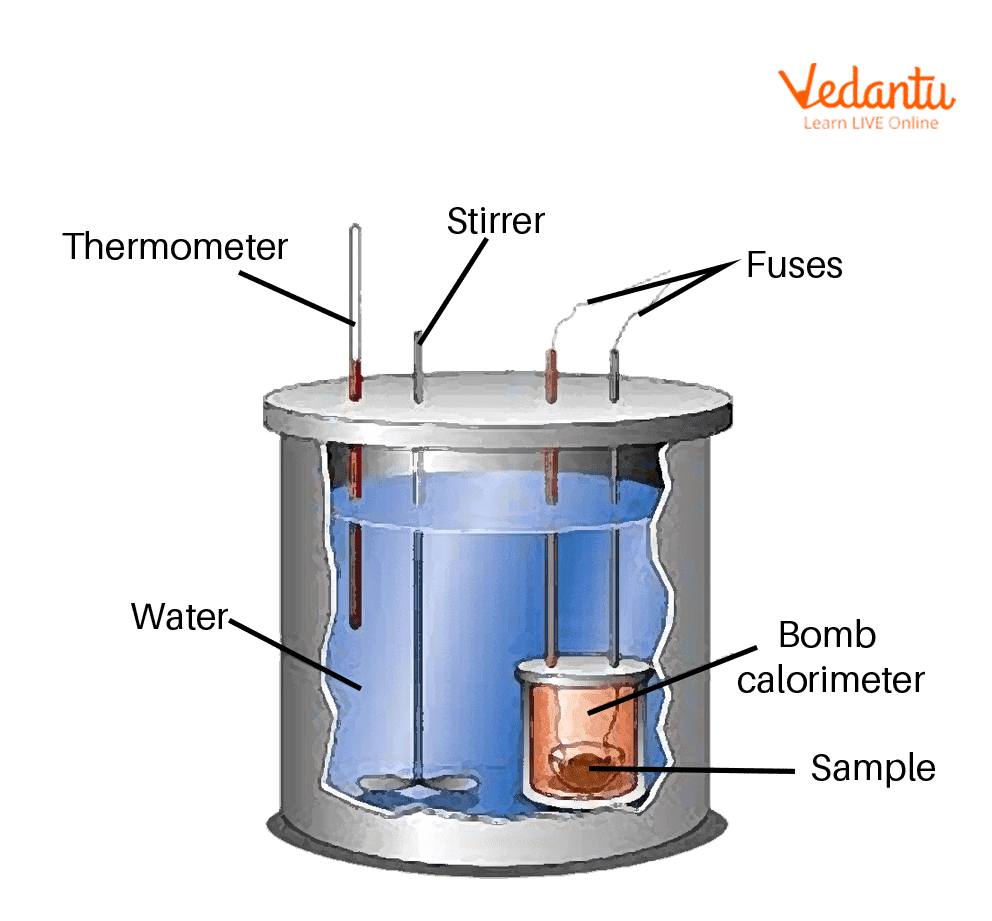
Bomb Calorimeter Learn Important Terms and Concepts
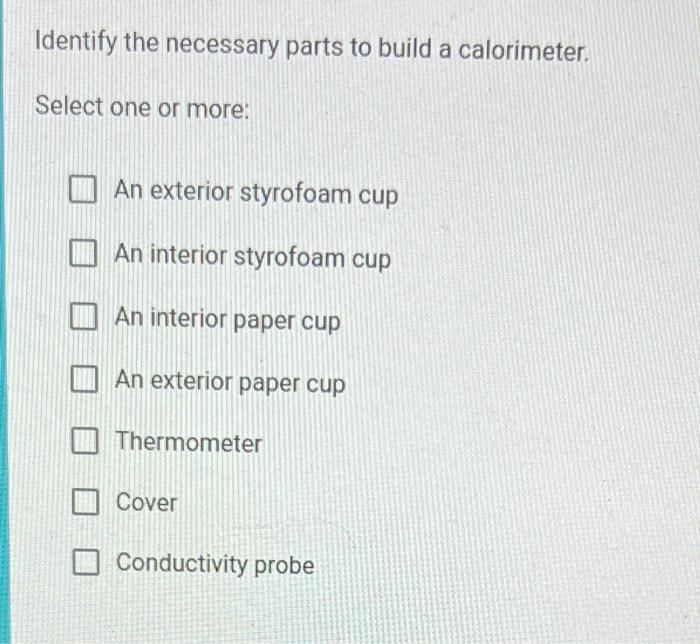
Solved Identify the necessary parts to build a calorimeter.
How To Build A Calorimeter
An Exterior Styrofoam Cup An Interior Styrofoam Cup An Interior Paper Cup An Exterior.
The Water In A Calorimeter Absorbing Heat Given Off By A Reaction Or Hot Object.
Two Versions Of The Recipe Are Provided For.
The Law Of Conservation Of Energy States That ______ An Example Of The Law In The Laboratory Is _______.
Related Post:
.png)