Reaction Builds Polymers From Monomers
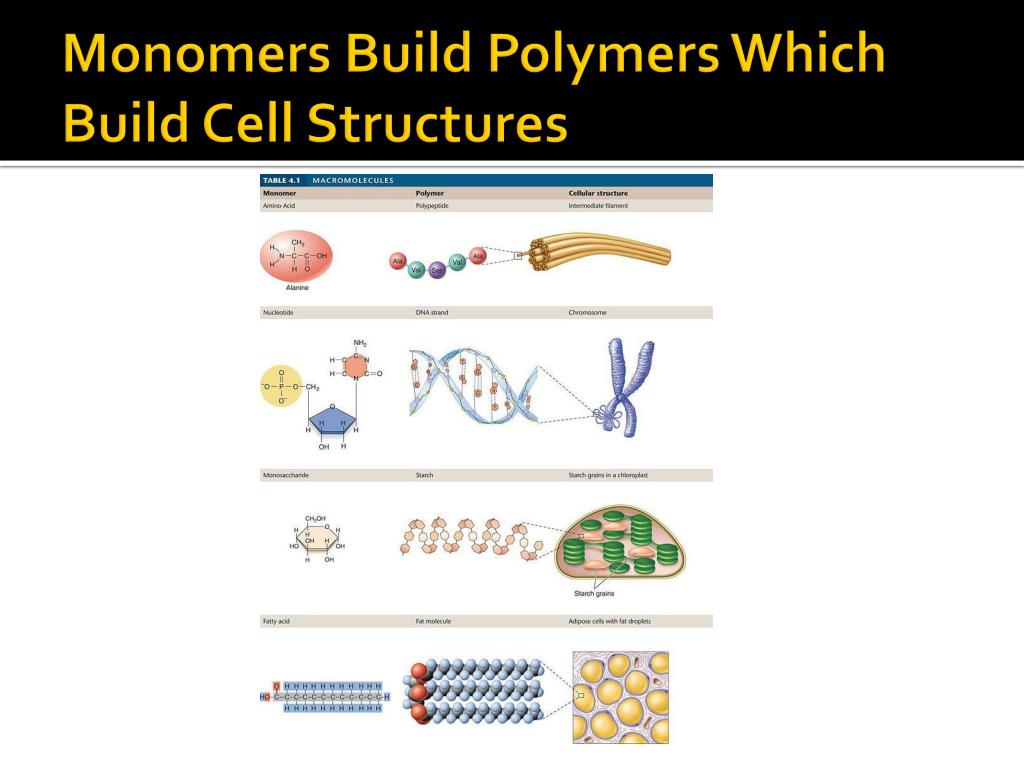
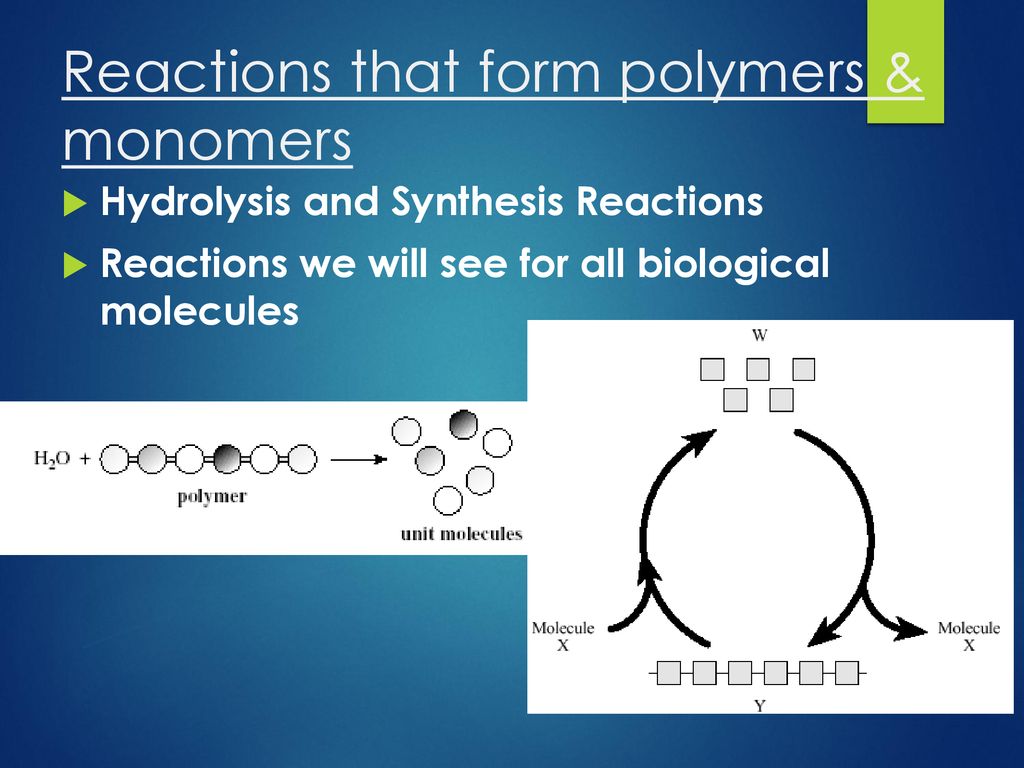
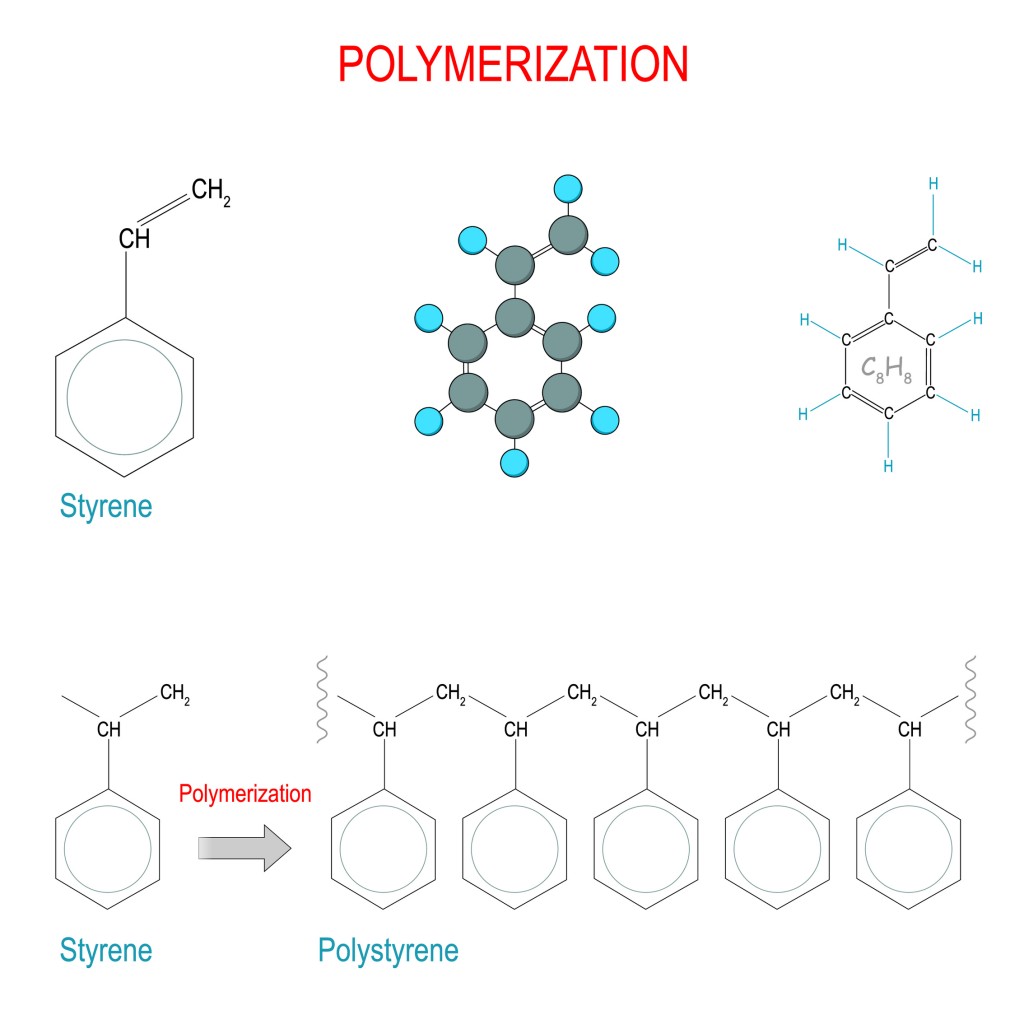
Reaction Builds Polymers From Monomers - Commonly found polymers include carbohydrates, lipids or proteins, and are all made of repeating monomer units. The conversion of the different terminal groups, which after assimilation into the polymer structure build part of the backbone, should be approximately the same. There are two main types of polymerization: Dehydration synthesis is a chemical reaction that forms covalent bonds between monomers to build polymers. There are two general types of polymerization reactions: In cells, polymers are created by linking monomers together. Water is added to cleave the covalent bonds between monomers in a. A hydrolysis reaction breaks down polymers into monomers. What is the role of water in a hydrolysis reaction? Carbohydrate monomers called monosaccharides are. An enzyme covalently bonds two. “it’s so easy,” dreiling said. Monomers are the fundamental building blocks of polymers. The researchers shredded synthetic or commercial polybutadiene and. During polymerization reactions, monomers undergo a series of chemical transformations, typically through the formation of. What is the name of the reaction involved in building polymers from monomers? The first polymer, the flexible one, retains a double bond that is needed to make the second, in a reaction initiated and controlled by light. There are two main types of polymerization: Water is added to cleave the covalent bonds between monomers in a. Study with quizlet and memorize flashcards containing terms like a dehydration reaction builds polymers from monomers., a hydrolysis reaction breaks down polymers into monomers, 3. Polymers are constructed from monomers through a process called polymerization. Deyhdration reactions are used to make larger molecules (polymers) from smaller molecules. Dehydration synthesis builds polymers by removing a water molecule to link. In addition polymerization, the monomers add to one. Polymers generally form either from an addition reaction or a condensation reaction. Polymers are constructed from monomers through a process called polymerization. Deyhdration reactions are used to make larger molecules (polymers) from smaller molecules. In cells, polymers are created by linking monomers together. A(n) amino acid is a building block of polypeptides, such as the protein hemoglobin. Monomers are the fundamental building blocks of polymers. An enzyme covalently bonds two. What is the role of water in a hydrolysis reaction? The researchers shredded synthetic or commercial polybutadiene and. Study with quizlet and memorize flashcards containing terms like a dehydration reaction builds polymers from monomers., a hydrolysis reaction breaks down polymers into monomers, 3. The conversion of the different terminal groups, which after assimilation into the. There are two main types of polymerization: Water is added to cleave the covalent bonds between monomers in a. During this process, a hydroxyl group (oh) from one monomer interacts with a. For example, poly(ethyleneterepthalate), a polyester known as pet that is commonly found in soda bottles, forms from a reaction of two monomers: A(n) amino acid is a building. Hydrolysis reactions use water to breakdown polymers into monomers and is the opposite of dehydration synthesis, which forms water when synthesizing a polymer from. Dna molecules are replicated in a cell by building new dna molecules from nucleotides. For example, poly(ethyleneterepthalate), a polyester known as pet that is commonly found in soda bottles, forms from a reaction of two monomers:. Water is added to cleave the covalent bonds between monomers in a. The first polymer, the flexible one, retains a double bond that is needed to make the second, in a reaction initiated and controlled by light. Polymers are constructed from monomers through a process called polymerization. Small molecules, called monomers, are assembled into long chains called polymers by a. Deyhdration reactions are used to make larger molecules (polymers) from smaller molecules. An enzyme covalently bonds two. Conspectusa holistic description of biopolymers and their evolutionary origins will contribute to our understanding of biochemistry, biology, the origins of life, and signatures of. Small molecules, called monomers, are assembled into long chains called polymers by a dehydration reaction. Let's look at this. What is the role of water in a hydrolysis reaction? Carbohydrate monomers called monosaccharides are. The first polymer, the flexible one, retains a double bond that is needed to make the second, in a reaction initiated and controlled by light. During this process, a hydroxyl group (oh) from one monomer interacts with a. Polymers generally form either from an addition. Polymers generally form either from an addition reaction or a condensation reaction. Let's look at this reaction more closely. Small molecules, called monomers, are assembled into long chains called polymers by a dehydration reaction. Study with quizlet and memorize flashcards containing terms like a dehydration reaction builds polymers from monomers., a hydrolysis reaction breaks down polymers into monomers, 3. Addition. The small molecules which make up polymers are called monomers. The two types of reactions are dehydration synthesis (also known as condensation) and hydrolysis. Study with quizlet and memorize flashcards containing terms like a dehydration reaction builds polymers from monomers., a hydrolysis reaction breaks down polymers into monomers, 3. Let's look at this reaction more closely. The monomers combine with. Study with quizlet and memorize flashcards containing terms like a dehydration reaction builds polymers from monomers., a hydrolysis reaction breaks down polymers into monomers, 3. Chemical reactions that build polymers from monomers are called dehydration reactions. Polymers generally form either from an addition reaction or a condensation reaction. The researchers shredded synthetic or commercial polybutadiene and. The monomers combine with each other using covalent bonds to form larger molecules known as. A(n) amino acid is a building block of polypeptides, such as the protein hemoglobin. During polymerization reactions, monomers undergo a series of chemical transformations, typically through the formation of. The two types of reactions are dehydration synthesis (also known as condensation) and hydrolysis. Monomers are the fundamental building blocks of polymers. The conversion of the different terminal groups, which after assimilation into the polymer structure build part of the backbone, should be approximately the same. A hydrolysis reaction breaks down polymers into monomers. Dna molecules are replicated in a cell by building new dna molecules from nucleotides. Water is added to cleave the covalent bonds between monomers in a. The small molecules which make up polymers are called monomers. The first polymer, the flexible one, retains a double bond that is needed to make the second, in a reaction initiated and controlled by light. Polymers are broken down into monomers in a process known as hydrolysis, which means “to split water,” a.Biochemical Reactions that Make and Break Molecules ppt download
PPT Organic Compounds PowerPoint Presentation, free download ID5463602
Chapter 3 The Molecules of Cells. ppt download
Vector Illustration Polymerization Reaction Conversion Monomers Stock
PPT BIOCHEMISTRY Biochemical processes are chemical reactions that
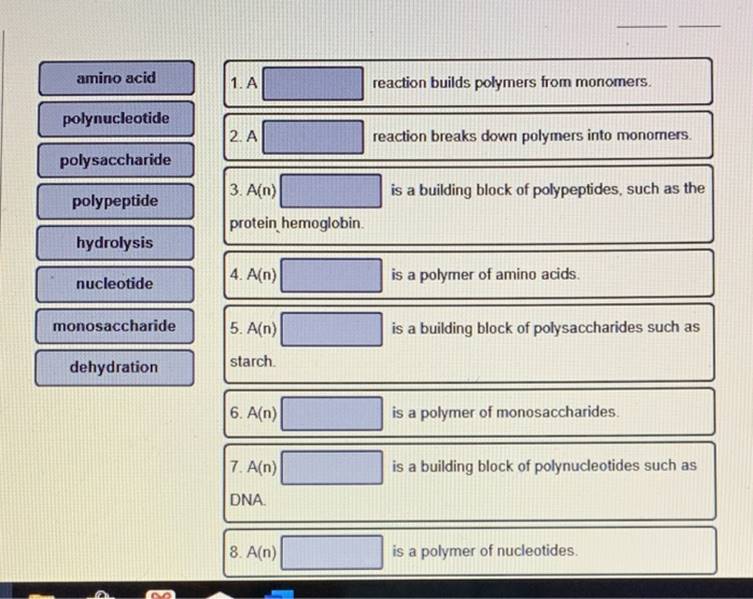
Solved A reaction builds polymers from monomers A reaction
Monomers And Polymers Definition, Chemical Properties And Differences
(Get Answer) Question Amino Acid Reaction Builds Polymers From
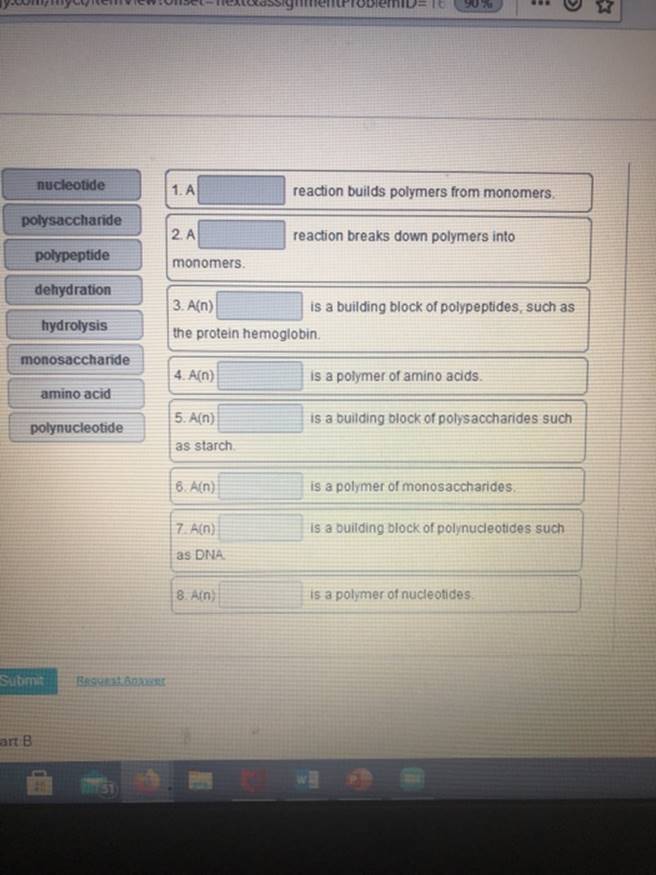
(Get Answer) 900 Nucleotide 1. A Reaction Builds Polymers From
Scientific Designing of Polymerization Reaction. Converting Monomers to
Addition Polymerization And Condensation Polymerization.
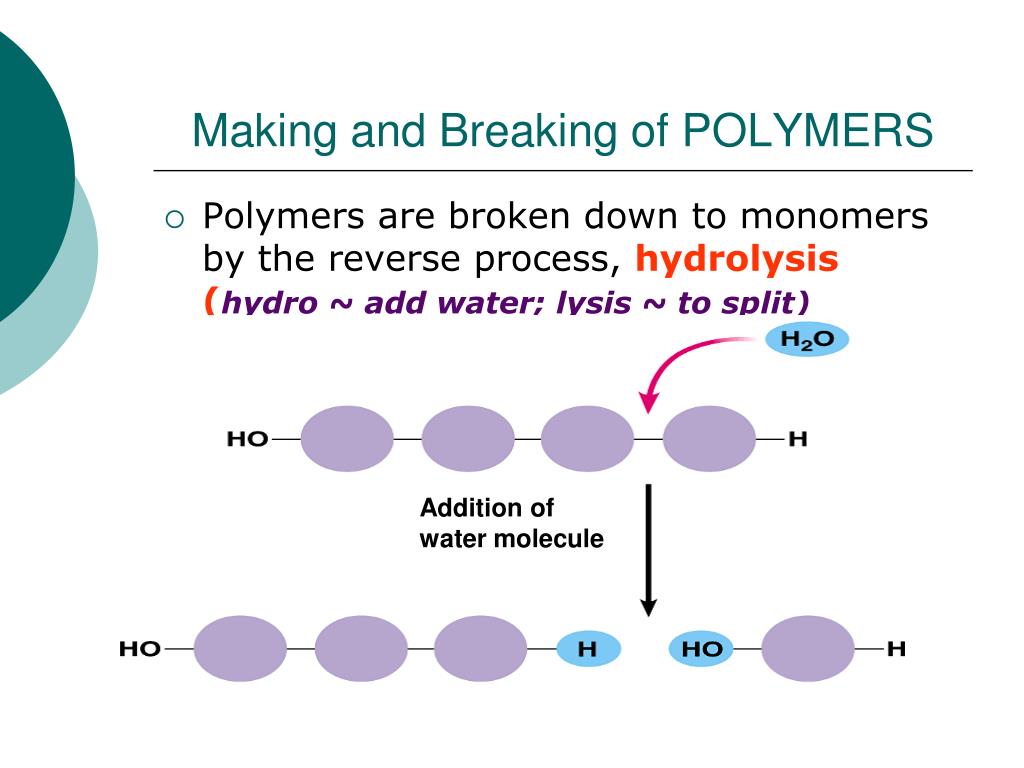
Polymers Are Broken Down Into Monomers In A Process Known As Hydrolysis, Which Means “To Split Water,” A Reaction In Which A Water Molecule Is Used During The Breakdown (Figure 3.1.2.
Conspectusa Holistic Description Of Biopolymers And Their Evolutionary Origins Will Contribute To Our Understanding Of Biochemistry, Biology, The Origins Of Life, And Signatures Of.
Dehydration Synthesis Builds Polymers By Removing A Water Molecule To Link.
Related Post: