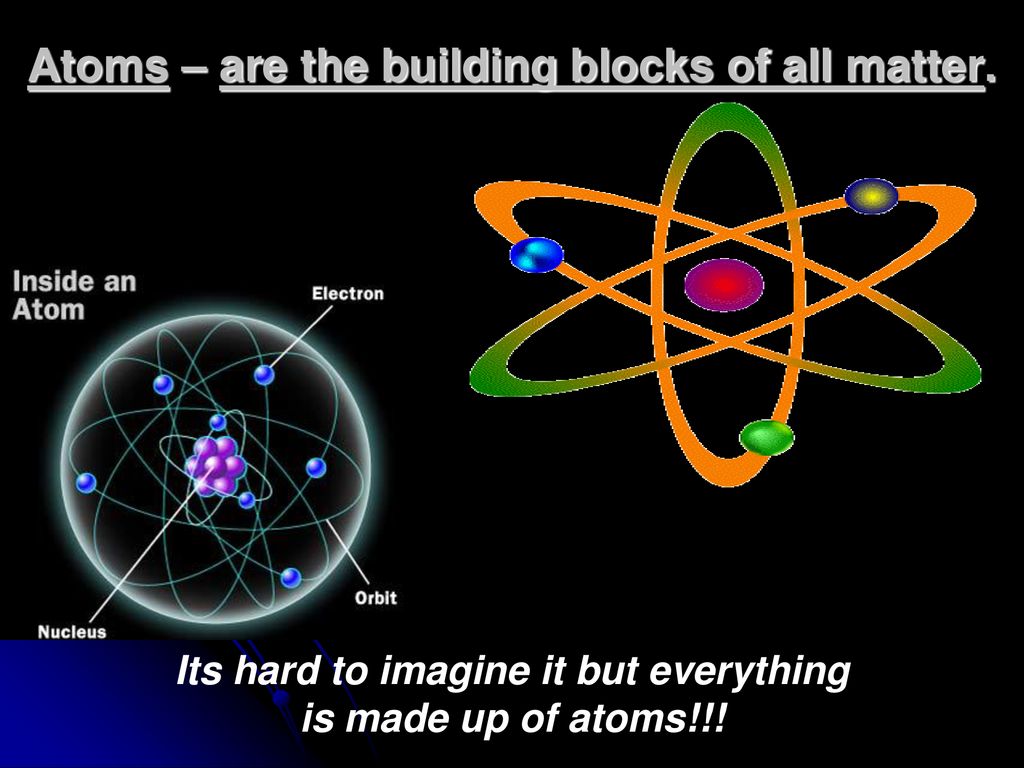
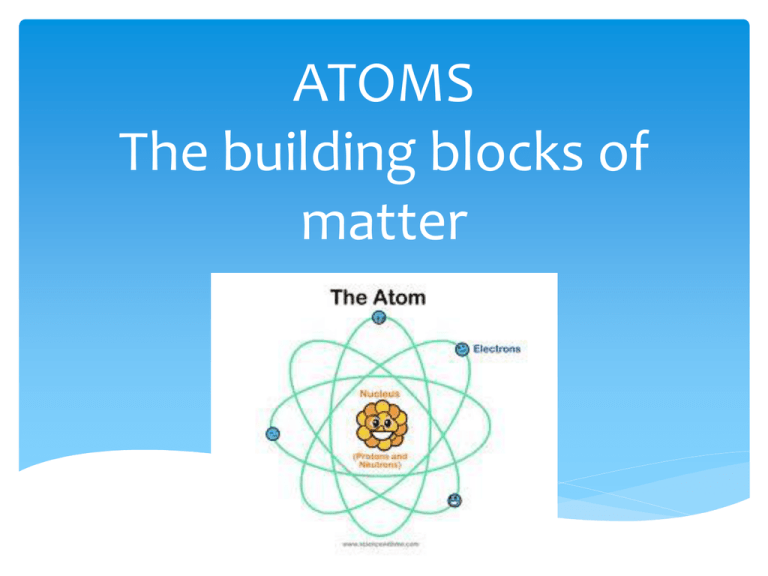
This Is The Basic Building Block Of All Matter
This Is The Basic Building Block Of All Matter - We consider the atomic theory as the foundation for understanding the interactions and changes in matter. It is made up of smaller particles called protons, neutrons, and electrons. They form the smallest unit of an element, retaining its unique properties, and are the building blocks of all matter in the universe. Scientists once thought the most fundamental building block of matter was a particle called the atom. All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. These basic building blocks lay the foundation for all of the ambitious projects detailed. These particles occur in two basic types called quarks and leptons. From atoms to quarks, we will discuss the structure and properties of each of these building blocks and how they come together to create the world around us. We will also delve into. These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. From atoms to quarks, we will discuss the structure and properties of each of these building blocks and how they come together to create the world around us. It is made up of smaller particles called protons, neutrons, and electrons. Despite their submicroscopic size, atoms hold. An element is a pure substance that is distinguished from all. These basic building blocks lay the foundation for all of the ambitious projects detailed. These particles occur in two basic types called quarks and leptons. Study with quizlet and memorize flashcards containing terms like atom, valence electrons, element and more. Trusted by teachersfree full access trialloved by kidseducational & fun All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. Atoms are the basic building blocks that are used for every type of matter in the known universe. Dramatic discoveries over the last century have completely changed our view of. All matter around us is made of elementary particles, the building blocks of matter. We will also delve into. Atoms are the basic building blocks that are used for every type of matter in the known universe. Trusted by teachersfree full access trialloved by kidseducational & fun All matter around us is made of elementary particles, the building blocks of matter. Now we know that the atom is made of many smaller pieces, known as subatomic. These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Study with quizlet and memorize flashcards containing terms like atom, valence electrons, element and. Scientists once thought the most fundamental building block of matter was a particle called the atom. All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. Each group consists of six particles, which. Atoms are the basic building blocks that are used for every type of matter in the known universe.. These basic building blocks lay the foundation for all of the ambitious projects detailed. Considered to be the basic building block of matter. Atoms are the basic building blocks that are used for every type of matter in the known universe. They are extremely small and are made up of even smaller particles. Now we know that the atom is. An element is a pure substance that is distinguished from all. They are extremely small and are made up of even smaller particles. Atoms are the basic building blocks that are used for every type of matter in the known universe. It is made up of smaller particles called protons, neutrons, and electrons. These particles occur in two basic types. We will also delve into. Each group consists of six particles, which. These basic building blocks lay the foundation for all of the ambitious projects detailed. These particles occur in two basic types called quarks and leptons. They are extremely small and are made up of even smaller particles. Atoms are the basic building blocks that are used for every type of matter in the known universe. Dramatic discoveries over the last century have completely changed our view of. These basic building blocks lay the foundation for all of the ambitious projects detailed. These particles occur in two basic types called quarks and leptons. Study with quizlet and memorize. From atoms to quarks, we will discuss the structure and properties of each of these building blocks and how they come together to create the world around us. We will also delve into. Despite their submicroscopic size, atoms hold. Each group consists of six particles, which. They are extremely small and are made up of even smaller particles. These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. They are extremely small and are made up of even smaller particles. They form the smallest unit of an element, retaining its unique properties, and are the building blocks of all matter in the universe. An element is a pure substance that is. We will also delve into. These particles occur in two basic types called quarks and leptons. Trusted by teachersfree full access trialloved by kidseducational & fun We consider the atomic theory as the foundation for understanding the interactions and changes in matter. These basic building blocks lay the foundation for all of the ambitious projects detailed. We consider the atomic theory as the foundation for understanding the interactions and changes in matter. Study with quizlet and memorize flashcards containing terms like atom, valence electrons, element and more. Atoms are the basic building blocks that are used for every type of matter in the known universe. Trusted by teachersfree full access trialloved by kidseducational & fun Dramatic discoveries over the last century have completely changed our view of. All matter around us is made of elementary particles, the building blocks of matter. Considered to be the basic building block of matter. All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. These particles occur in two basic types called quarks and leptons. They are extremely small and are made up of even smaller particles. From atoms to quarks, we will discuss the structure and properties of each of these building blocks and how they come together to create the world around us. Scientists once thought the most fundamental building block of matter was a particle called the atom. They form the smallest unit of an element, retaining its unique properties, and are the building blocks of all matter in the universe. In this unit, we shall explore particle physics, the study of the fundamental constituents of matter. An element is a pure substance that is distinguished from all. Additionally, we discuss isotopes and draw electron configurations as well as find the.Basic Chemistry Section 2.1 (Matter). ppt download
8+ Chapter 3 Review Atoms The Building Blocks Of Matter LialaTierney
Building blocks of matter g3
ATOMS The building blocks of matter
ATOMS Basic building blocks of all matter. ppt video online download
PPT What are the basic building blocks of matter? PowerPoint
PPT CHAPTER 3 Atoms The Building Blocks of Matter PowerPoint
PPT Ch. 3 Atoms The Building Blocks of Matter PowerPoint
PPT Chemistry PowerPoint Presentation, free download ID9706406
PPT I. What is an atom? PowerPoint Presentation, free download ID
Despite Their Submicroscopic Size, Atoms Hold.
We Will Also Delve Into.
Now We Know That The Atom Is Made Of Many Smaller Pieces, Known As Subatomic.
Each Group Consists Of Six Particles, Which.
Related Post: