What Are Building Blocks Of Matter
What Are Building Blocks Of Matter - Matter occupies space and has mass. Sample exam questions covering the building blocks of science, with advice for multiple choice, structured, mathematical and practical questions. In the 1800's one of the principle proponents of the developing atomic theory was john dalton. Our everyday world is made of just three of these building blocks: At its most fundamental level, life is made up of matter. In this unit, we shall explore particle physics, the study of the fundamental constituents of matter. The proton, neutron, and electron. Atoms are the basic building blocks that are used for every type of matter in the known universe. You learned earlier that all matter in the universe is made out of tiny building blocks called atoms. Bitesize has lots of general aqa synergy. At its most fundamental level, life is made up of matter. Atoms are made up of even smaller subatomic particles, three types of which are important: Discuss the relationships between matter, mass, elements, compounds, atoms, and subatomic particles; Combine the results of these two 20th century revolutions into a single theory which describes the interactions of the fundamental building blocks of nature. Everything in the universe, except energy, is made up of atoms. You learned earlier that all matter in the universe is made out of tiny building blocks called atoms. Matter occupies space and has mass. Our everyday world is made of just three of these building blocks: What are the building blocks of matter? Bitesize has lots of general aqa synergy. The standard model explains how the basic building blocks of matter interact, governed by four fundamental forces. Sample exam questions covering the building blocks of science, with advice for multiple choice, structured, mathematical and practical questions. Bitesize has lots of general aqa synergy. These basic building blocks lay the foundation for all of the ambitious projects detailed. All matter is. The proton, neutron, and electron. What are the building blocks of matter? You learned earlier that all matter in the universe is made out of tiny building blocks called atoms. Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. The standard model explains how the basic building blocks of matter. The proton, neutron, and electron. Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. He advanced the idea that all atoms of a particular element are identical (as far. In this unit, we shall explore particle physics, the study of the fundamental constituents of matter. What are the building blocks. Physicists have identified 12 building blocks that are the fundamental constituents of matter. Atoms, the fundamental building blocks of all matter, are composed of three essential subatomic particles: The standard model explains how the basic building blocks of matter interact, governed by four fundamental forces. Within the chemical world, atoms are the essential building blocks, composing the many molecules and. Distinguish between atomic number and mass number; Everything in the universe, except energy, is made up of atoms. In this unit, we shall explore particle physics, the study of the fundamental constituents of matter. All modern scientists accept the concept of the atom, but when the concept of. Atoms, the fundamental building blocks of all matter, are composed of three. The proton, neutron, and electron. He advanced the idea that all atoms of a particular element are identical (as far. Discuss the relationships between matter, mass, elements, compounds, atoms, and subatomic particles; All matter is composed of elements, substances that cannot be broken down or. Physicists have identified 12 building blocks that are the fundamental constituents of matter. Atoms are made up of even smaller subatomic particles, three types of which are important: These basic building blocks lay the foundation for all of the ambitious projects detailed. Atoms, the fundamental building blocks of all matter, are composed of three essential subatomic particles: The proton, neutron, and electron. Atoms are made up of even smaller subatomic particles, three types. All atoms consist of a small, positively charged. In this unit, we shall explore particle physics, the study of the fundamental constituents of matter. Everything in the universe, except energy, is made up of atoms. All matter is composed of elements, substances that cannot be broken down or. Atoms are made up of even smaller subatomic particles, three types of. The proton, neutron, and electron. All atoms consist of a small, positively charged. Sample exam questions covering the building blocks of science, with advice for multiple choice, structured, mathematical and practical questions. Combine the results of these two 20th century revolutions into a single theory which describes the interactions of the fundamental building blocks of nature. What are the building. Atoms are made up of even smaller subatomic particles, three types of which are important: Discuss the relationships between matter, mass, elements, compounds, atoms, and subatomic particles; The standard model explains how the basic building blocks of matter interact, governed by four fundamental forces. What are the building blocks of matter? Sample exam questions covering the building blocks of science,. Distinguish between atomic number and mass number; In the 1800's one of the principle proponents of the developing atomic theory was john dalton. Discuss the relationships between matter, mass, elements, compounds, atoms, and subatomic particles; At its most fundamental level, life is made up of matter. All modern scientists accept the concept of the atom, but when the concept of. The standard model explains how the basic building blocks of matter interact, governed by four fundamental forces. The proton, neutron, and electron. Everything in the universe, except energy, is made up of atoms. Distinguish between atomic number and mass number; Atoms are made up of even smaller subatomic particles, three types of which are important: You learned earlier that all matter in the universe is made out of tiny building blocks called atoms. All atoms consist of a small, positively charged. Discuss the relationships between matter, mass, elements, compounds, atoms, and subatomic particles; Combine the results of these two 20th century revolutions into a single theory which describes the interactions of the fundamental building blocks of nature. All matter is composed of elements, substances that cannot be broken down or. These basic building blocks lay the foundation for all of the ambitious projects detailed.PPT Chapter 1 Introduction Matter and Measurement PowerPoint
8+ Chapter 3 Review Atoms The Building Blocks Of Matter LialaTierney
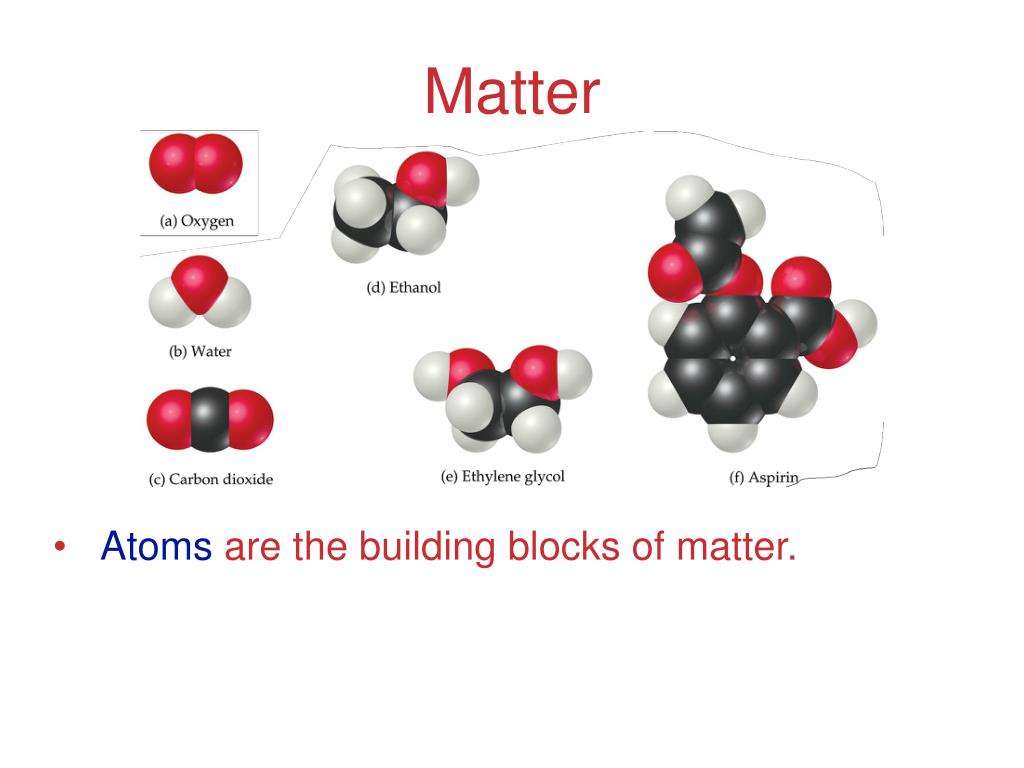
PPT CHAPTER 3 Atoms The Building Blocks of Matter PowerPoint
Building blocks of matter g3
PPT ATOMS The building blocks of matter PowerPoint Presentation, free
Building Blocks of Matter YouTube
PPT Atoms, Ions and Molecules The Building Blocks of Matter
PPT The Building Blocks of Matter Atoms PowerPoint Presentation
PPT Ch. 3 Atoms The Building Blocks of Matter PowerPoint
Chapter 1 Matter Matter anything that has mass and takes up space
Bitesize Has Lots Of General Aqa Synergy.
Atoms Are The Basic Building Blocks That Are Used For Every Type Of Matter In The Known Universe.
Physicists Have Identified 12 Building Blocks That Are The Fundamental Constituents Of Matter.
The Proton, Neutron, And Electron.
Related Post: